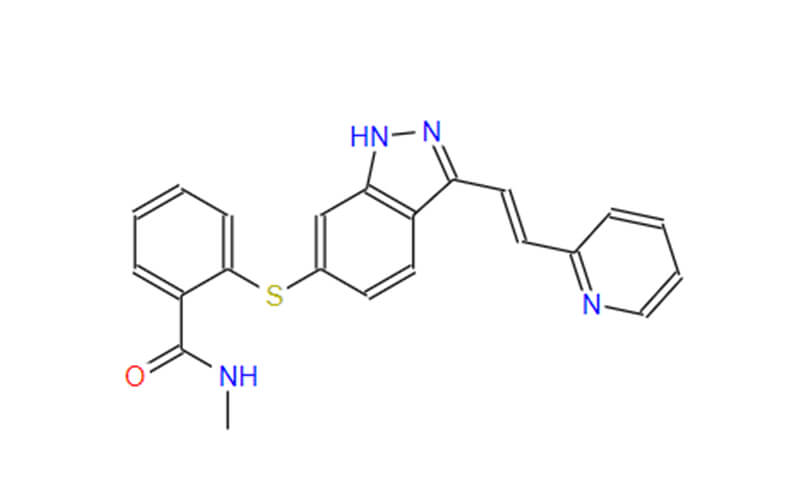
Oclacitinib API: An Overview for Veterinary and Human Applications
Oclacitinib, a Janus kinase (JAK) inhibitor, has emerged as a prominent therapeutic agent in veterinary medicine, particularly for managing atopic dermatitis in dogs. Its active pharmaceutical ingredient (API), oclacitinib maleate, exhibits unique properties and holds promising potential for human applications. This article delves into the intricate details of oclacitinib API, exploring its chemical characteristics, mechanism of action, and diverse applications, and further examines the specific formulation, oclacitinib maleate, its advantages, and its usage in both veterinary and human contexts.
Oclacitinib API: Chemical and Pharmacological Intricacies
Oclacitinib, a pyrrolopyrimidine derivative, boasts a unique chemical structure that underpins its potent inhibitory activity against Janus kinases (JAKs). These enzymes serve as pivotal regulators within the JAK-STAT (Janus kinase-signal transducer and activator of transcription) signaling pathway, a cornerstone of the immune system. Oclacitinib’s selectivity towards JAK1 and JAK3 translates to effective modulation of inflammatory cascades, ultimately alleviating the clinical manifestations of various inflammatory disorders.
The intricate mechanism of action hinges on oclacitinib’s competitive binding to the ATP-binding pocket of JAK enzymes. This interaction effectively impedes the phosphorylation of STAT proteins, crucial downstream signaling molecules responsible for perpetuating inflammatory responses. Consequently, oclacitinib downregulates the expression of pro-inflammatory cytokines and chemokines, culminating in a significant reduction in inflammation and tissue damage.
Diving Deeper into the Chemical Landscape
- Structural Specificity: Oclacitinib’s pyrrolopyrimidine core, adorned with specific functional groups, enables precise interactions with the ATP-binding pocket of JAK enzymes. This selectivity minimizes off-target effects, contributing to its favorable safety profile.
- Binding Affinity: High-affinity binding between oclacitinib and JAKs ensures potent inhibition, even at low concentrations. This translates to effective clinical outcomes with minimal drug requirements.
- Pharmacokinetic Properties: Understanding oclacitinib’s absorption, distribution, metabolism, and excretion (ADME) profile is crucial for optimizing drug delivery and ensuring systemic exposure at therapeutic levels.
Unlocking the Pharmacological Potential
- JAK-STAT Pathway Modulation: Oclacitinib’s targeted inhibition of JAK1 and JAK3 disrupts the phosphorylation and activation of STAT proteins, effectively dampening the expression of pro-inflammatory genes. This comprehensive modulation of the JAK-STAT pathway contributes to its therapeutic efficacy.
- Cytokine and Chemokine Regulation: By suppressing the production of pro-inflammatory mediators like IL-6, IL-31, and CCL2, oclacitinib effectively dampens inflammatory processes at multiple levels. This multifaceted approach contributes to its broad therapeutic potential.
- Disease-Specific Applications: Understanding the specific role of JAK-STAT signaling in various inflammatory disorders, such as atopic dermatitis and alopecia areata, is crucial for harnessing oclacitinib’s therapeutic potential in targeted clinical settings.

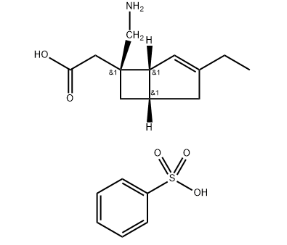
Oclacitinib Maleate: Unveiling the Advantages of the Salt Form for Pharmaceutical Applications
Oclacitinib maleate, the salt form of oclacitinib, reigns supreme as the preferred formulation for both veterinary and, in ongoing investigations, human therapeutic applications. Its reign stems from several key advantages it possesses over the free base form, particularly in terms of its physicochemical properties and suitability for pharmaceutical formulations.
1. Enhanced Solubility: Overcoming Aqueous Obstacles
The free base form of oclacitinib exhibits limited aqueous solubility, posing a significant challenge for formulating effective oral medications. Oclacitinib maleate, however, readily dissolves in water, overcoming this hurdle and paving the way for efficient absorption into the bloodstream. This enhanced solubility translates to improved bioavailability, ensuring a greater proportion of the drug reaches its site of action, ultimately maximizing therapeutic efficacy.
2. Bioavailability Boost: Optimizing Drug Delivery
Bioavailability refers to the fraction of an administered drug that reaches systemic circulation and exerts its intended effect. Oclacitinib maleate demonstrates superior bioavailability compared to the free base form, signifying a larger portion of the drug is available to elicit its therapeutic response. This translates to potentially lower required dosages, potentially minimizing adverse effects and optimizing therapeutic outcomes.
3. Pharmaceutical Elegance: Tailoring for Diverse Formulations
Oclacitinib maleate’s favorable physicochemical properties, including improved solubility and stability, make it highly amenable to various pharmaceutical formulations. This versatility allows for the development of diverse dosage forms tailored to specific patient needs and preferences. Tablets, chewable tablets, and potentially even liquid formulations become feasible with oclacitinib maleate, catering to a broader patient population and enhancing treatment adherence.
4. Oral Administration Convenience: A Patient-Centric Approach
Oral administration remains the preferred route for most medications due to its ease of use and non-invasive nature. Oclacitinib maleate’s suitability for oral formulations aligns perfectly with this preference, offering a convenient and patient-friendly administration method. This can significantly improve treatment compliance and adherence, ultimately contributing to better therapeutic outcomes.
5. Regulatory Acceptance: Paving the Path to Clinical Use
Regulatory agencies, such as the FDA and EMA, meticulously evaluate the safety, efficacy, and quality of pharmaceutical formulations before approving them for clinical use. Oclacitinib maleate’s well-defined chemical structure, stability, and established manufacturing processes facilitate its compliance with stringent regulatory requirements. This paves the way for its wider clinical adoption and potential benefit for patients suffering from various inflammatory conditions.

Oclacitinib Maleate for Dogs: A Targeted Therapy for Atopic Dermatitis
Oclacitinib maleate has emerged as a cornerstone therapy in the veterinary management of atopic dermatitis, a chronic, pruritic skin condition significantly impacting canine quality of life. Its efficacy in controlling pruritus and alleviating skin lesions has demonstrably improved the well-being of affected dogs.
1. Addressing the Pathophysiology of Atopic Dermatitis
Atopic dermatitis is a complex, multifactorial inflammatory skin disease characterized by IgE-mediated hypersensitivity reactions. Oclacitinib’s mechanism of action specifically targets JAK1 and JAK3, key enzymes within the JAK-STAT signaling pathway, which plays a pivotal role in mediating inflammatory responses. By selectively inhibiting these enzymes, oclacitinib effectively downregulates the production of pro-inflammatory cytokines and chemokines, leading to reduced inflammation and itch.
2. Clinical Efficacy and Evidence-Based Support
Numerous clinical trials and field studies have established the efficacy and safety of oclacitinib maleate in managing canine AD. These studies have demonstrated significant improvements in pruritus scores, lesion severity, and overall quality of life in affected dogs. Additionally, oclacitinib has been shown to be more effective than glucocorticoids, a commonly used treatment for atopic dermatitis, in terms of long-term disease control and reducing the risk of adverse effects.
3. Dosage Regimens and Tailored Treatment
Oclacitinib maleate is available in various commercially available veterinary products, each adhering to stringent regulatory guidelines and quality control standards. The recommended dosage varies depending on the dog’s weight and the severity of the condition. Once-daily administration is typically sufficient, with initial loading doses sometimes employed for rapid symptom control. Veterinarians play a crucial role in tailoring treatment plans based on individual needs and monitoring for potential side effects, such as vomiting, diarrhea, and otitis externa, which are generally mild and transient.
4. Beyond Symptom Control: Advancing Canine Well-being
The impact of atopic dermatitis extends beyond pruritus and skin lesions. Chronic inflammation and discomfort can significantly affect a dog’s quality of life, leading to anxiety, sleep disturbances, and reduced socialization. Oclacitinib’s ability to effectively control these symptoms not only improves physical well-being but also enhances the emotional and behavioral well-being of affected dogs, fostering stronger human-animal bonds.
5. Future Directions and Ongoing Research
While oclacitinib has established itself as a valuable tool in managing canine atopic dermatitis, ongoing research continues to explore its potential applications and optimize its use. Studies are investigating the long-term safety and efficacy of oclacitinib, its potential combination with other therapies, and its role in preventing disease flare-ups. Additionally, research is exploring the use of oclacitinib for other canine inflammatory skin conditions, potentially expanding its therapeutic reach.

Oclacitinib Maleate for Humans: Unveiling the Potential Beyond Veterinary Applications
While currently confined to the veterinary realm, the therapeutic potential of oclacitinib is beckoning the frontiers of human medicine. Ongoing clinical trials are actively exploring its efficacy in a spectrum of inflammatory conditions, including alopecia areata, vitiligo, and ulcerative colitis, offering a glimmer of hope for patients suffering from these debilitating ailments. However, before fully embracing its therapeutic potential in humans, further research is paramount to elucidate its safety and efficacy profile, paving the way for potential regulatory approval.
1. Expanding the Therapeutic Horizon
The success of oclacitinib in managing canine atopic dermatitis has ignited interest in its potential application to human inflammatory disorders. The shared involvement of the JAK-STAT pathway in the pathogenesis of various human inflammatory conditions, including alopecia areata, vitiligo, and ulcerative colitis, presents a compelling rationale for exploring oclacitinib’s therapeutic potential in these contexts.
2. Promising Preliminary Findings
Early-stage clinical trials have yielded encouraging results, hinting at the potential efficacy of oclacitinib in human applications. Studies in alopecia areata patients have shown promising improvements in hair regrowth, suggesting its ability to modulate the inflammatory cascade implicated in hair loss. Similarly, initial trials in vitiligo patients have demonstrated repigmentation, potentially offering a novel therapeutic avenue for this challenging condition. Additionally, research exploring the use of oclacitinib in ulcerative colitis patients suggests its potential to alleviate symptoms and promote mucosal healing.
3. Rigorous Research: Navigating the Path to Human Use
Despite the promising early results, comprehensive and rigorous research is paramount before oclacitinib can be considered for widespread human use. Thorough clinical trials, encompassing larger patient populations and longer durations, are essential to definitively assess its efficacy and safety profile in humans. Additionally, pharmacokinetic and pharmacodynamic studies are crucial to optimize dosage regimens and ensure adequate drug exposure for optimal therapeutic effect.
4. Safety Considerations: Prioritizing Patient Well-being
While oclacitinib has demonstrated a favorable safety profile in veterinary applications, meticulous evaluation of its safety in humans remains paramount. Potential side effects, including infections, gastrointestinal disturbances, and hematologic changes, require close monitoring and thorough investigation. Additionally, drug-drug interactions and potential risks associated with long-term use necessitate careful consideration.
5. Regulatory Hurdles: Paving the Path for Approval
Before oclacitinib can become a mainstream therapeutic option for human patients, stringent regulatory hurdles must be cleared. Regulatory agencies like the FDA and EMA demand comprehensive preclinical and clinical data demonstrating its safety, efficacy, and quality for human use. This process might necessitate additional clinical trials, rigorous manufacturing standards, and extensive safety assessments.
6. A Beacon of Hope for Patients in Need
Oclacitinib represents a beacon of hope for patients suffering from various inflammatory conditions with limited treatment options or experiencing inadequate response to existing therapies. The early clinical data coupled with its targeted mechanism of action paint a promising picture for its potential to improve patient outcomes. However, rigorous research and careful consideration of safety remain paramount before oclacitinib can become a reality for patients in need.
Why Choose Qingmu as Your Oclacitinib Manufacturer?
Choosing a reliable and qualified manufacturer for Oclacitinib API (Active Pharmaceutical Ingredient) is a critical decision for pharmaceutical companies and contract development and manufacturing organizations (CDMOs). There are some of Qingmu’s strengths and capabilities, demonstrating why it stands out as a strategic partner for your Oclacitinib needs.
1. Established Expertise in Oclacitinib Production
Qingmu boasts extensive experience in Oclacitinib API manufacturing, possessing a deep understanding of the intricate production processes and stringent quality control measures necessary for consistent and reliable drug production. This expertise translates to minimized development timelines, reduced regulatory hurdles, and enhanced product quality.
2. State-of-the-art Manufacturing Facilities
Qingmu operates cutting-edge manufacturing facilities equipped with advanced technology and instrumentation, ensuring adherence to cGMP (Current Good Manufacturing Practice) guidelines and international quality standards. This robust infrastructure guarantees the production of high-purity, contaminant-free Oclacitinib API, meeting the strictest regulatory requirements.
3. Stringent Quality Control Measures
Qingmu prioritizes rigorous quality control throughout the entire production process, implementing a multi-layered quality management system (QMS). This system encompasses meticulous raw material testing, in-process controls, and comprehensive final product analysis using sophisticated analytical techniques. This unwavering commitment to quality ensures the consistent production of safe and efficacious Oclacitinib API.
4. Regulatory Compliance and Global Reach
Qingmu maintains regulatory compliance with stringent global standards, including the US FDA, European EMA, and other relevant regulatory bodies. This ensures seamless regulatory navigation and expedites approval processes for Oclacitinib API, facilitating market access across various regions.
5. Competitive Pricing and Flexible Solutions
Qingmu offers competitive pricing models tailored to your specific needs and project requirements. Additionally, its flexible manufacturing capabilities can accommodate diverse production volumes and provide customized solutions to meet your unique project demands.
6. Collaborative Partnerships and Technical Support
Qingmu fosters collaborative partnerships with clients, offering dedicated technical support throughout the entire development and manufacturing process. This collaborative approach ensures clear communication, timely problem-solving, and efficient project execution.
7. Continuous Innovation and Sustainability
Qingmu remains committed to continuous innovation and process optimization, investing in research and development to enhance its manufacturing capabilities and sustainability practices. This dedication ensures the production of high-quality, environmentally friendly Oclacitinib API, aligning with your ethical and environmental values.
By carefully considering these factors, you can make an informed decision when selecting your Oclacitinib API manufacturing partner. Qingmu’s established expertise, robust infrastructure, stringent quality control, regulatory compliance, competitive pricing, and collaborative approach position it as a compelling choice for your Oclacitinib needs.

In conclusion, oclacitinib maleate stands as a testament to the power of salt formation in optimizing drug delivery. Its enhanced solubility, bioavailability, and suitability for oral formulations position it as the preferred choice for both veterinary and potential human applications. As research continues to explore the therapeutic potential of oclacitinib, oclacitinib maleate remains poised to play a pivotal role in advancing its clinical utility.