Ubenimex API
| CAS No. | 58970-76-6 |
| Therapeutic Category | Anti-Cancer/ Oncolog |
| Technology | Synthetic |
| Dosage Form | Oral Solids |
| Innovator Brand | bestatin(Japan) |
| Registration Status | China: A, GMP Japan: 229MF10025 Others: DMF |
| Polymorph | N/A |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
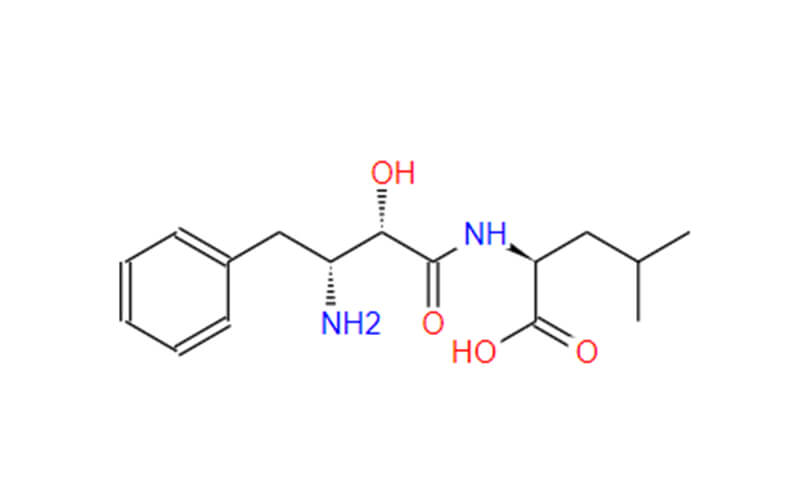
- Chemical Name: 2-(3-Amino-2-hydroxy-4-phenyl-butyrylamino)-4-methyl-pentanoic acid
- Molecular Formula: C16H24N2O4
- Molecular Weight: 308.373
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Ubenimex API
- Ubenimex (also known as bestatin) is a competitive aminopeptidase B inhibitor with an IC50 of 100 mg/ml for K562 cells. Proliferation of all the cell lines except KG1 was inhibited by bestatin. P39/TSU, HL60, and U937 were highly sensitive, with 50% growth inhibitory concentration. It is useful in the treatment of non-lymphocytic leukemia. In other types of cancers, ubenimex added to radiotherapy or chemotherapy following surgery significantly inmases survival time through immunopotentiation. It is being studied for use in the treatment of acute myelocytic leukemia.
Why Choose Us as Your Ubenimex Manufacturer?
- Qingmu’s Ubenimex has been approved in China(DMF filed and listed on CDE’s website) with GMP certificate and also filed MF in Japan PMDA, DMF in CTD format is available and can be supported for registration worldwide.
- Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, no risk to remove factory. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) and also customer audits from Europe, USA and Japan, etc.
- As a China Ubenimex manufacturer, Qingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.