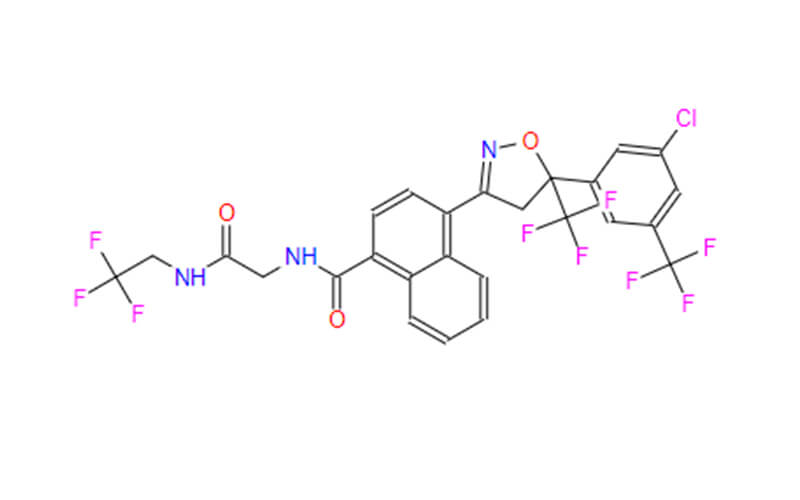
Afoxolaner API
| CAS No. | 1093861-60-9 |
| Therapeutic Category | Antiparasitics |
| Technology | Synthetic |
| Dosage Form | Oral Solids |
| Innovator Brand | Nexgard, Frontpro, and Nexgard Spectra(USA) |
| Registration Status | US: VMF006618 Others: VMF |
| Polymorph | Form A |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
- ChemicalName: 4-[(5R)-5-[3-chloro-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-4H-1,2-oxazol-3-yl]-N-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]naphthalene-1-carboxamide
- Molecular Formula: C26H17ClF9N3O3
- Molecular Weight: 625.87000
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 2.0%
- Purity: not less than 98%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement.
Applications of Afoxolaner API
- Afoxolaner (brand name NexGard®) is used to treat and control flea and tick infestations in dogs. After being ingested by a dog, afoxolaner is distributed throughout the dog’s body. When fleas or ticks bite the dog, they are exposed to the drug and killed during their blood meal.
- It acts as an antagonist at ligand-gated chloride channels, in particular those gated by the neurotransmitter gamma-aminobutyric acid (GABA-receptors). Isoxazolines, among the chloride channel modulators, bind to a distinct and unique target site within the insect GABA-gated chloride channels, thereby blocking the pre- and post-synaptic transfer of chloride ions across cell membranes. Prolonged afoxolaner-induced hyperexcitation results in uncontrolled activity of the central nervous system and death of insects and acarines.
Why Choose Us as Your Afoxolaner Manufacturer?
- As an Afoxolaner manufacturer, Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, and no risk of removing the factory.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. We also passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) and also customer audits from Europe, USA and Japan, etc. So please rest assured to choose us as your partner.
- Qingmu is an afoxolaner supplier, that successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.