Maropitant Base API
| CAS No. | 147116-67-4 |
| Therapeutic Category | Antiemetic |
| Technology | Synthetic |
| Dosage Form | Injection |
| Innovator Brand | Cerenia (USA) |
| Registration Status | VMF |
| Polymorph | Form B; New form with own patent |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
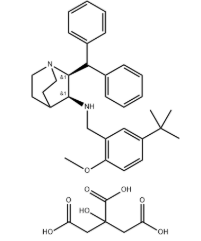
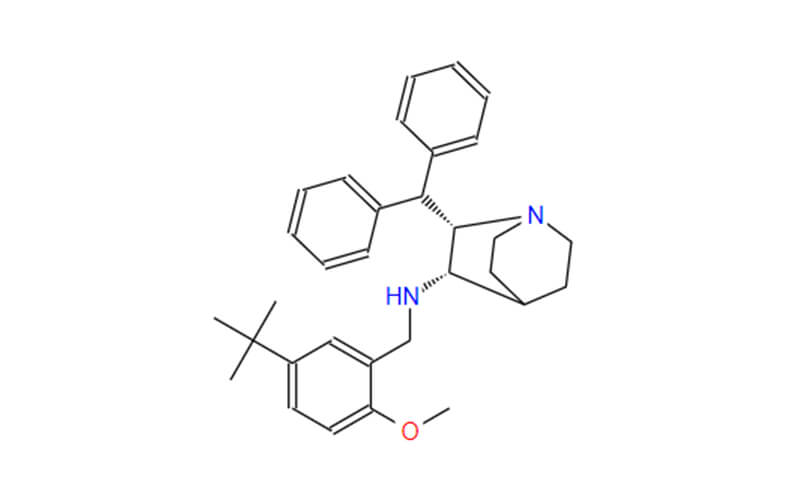
- Chemical Name:(2S,3S)-2-benzhydryl-N-[(5-tert-butyl-2-methoxyphenyl)methyl]-1-azabicyclo[2.2.2]octan-3-amine
- Molecular Formula:C32H40N2O
- Molecular Weight:468.67300
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 2.0%
- Purity: not less than 98%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Maropitant
- Injectable maropitant is used in dogs to treat and prevent acute vomiting; tablets are used for preventing vomiting from a variety of causes, though they require a higher dose to prevent vomiting from motion sickness. The injectable version is also licensed for preventing and treating acute vomiting in cats.
- Maropitant is effective in treating vomiting from a variety of causes, including gastroenteritis, chemotherapy, and kidney failure; when given beforehand, it can prevent vomiting caused by using an opioid as a premedication. Some have claimed that maropitant is better at treating vomiting than nausea, pointing to cats with chronic kidney disease who, when receiving maropitant, had a reduction in vomiting but no corresponding increase in appetite.
- Maropitant has been used in acute cases of rapid or labored breathing to prevent vomiting that could lead to aspiration pneumonia. It has been given in combination with a benzodiazepine to cats prior to stressful events (such as a veterinary visit) to possibly relieve hypersensitivity.
Why Choose Us as Your Maropitant Supplier?
- DMF in CTD format is available and can be supported for registration worldwide.
- As a Maropitant manufacturer, Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, and no risk of removing the factory. Our new API factory is under design and is predicted to be put into use in 2025.
- Qingmu is a maropitant factory, our team has rich experience in patent challenges on crystalline form & synthesis processes, synthetic route development, and scale-up & quality research.
- Our lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) as well as customer audits from Europe, USA, Japan, etc. So you can choose us with confidence.
- Having over a decade of experience, Qingmu is one of the leading maropitant suppliers in China. We successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.