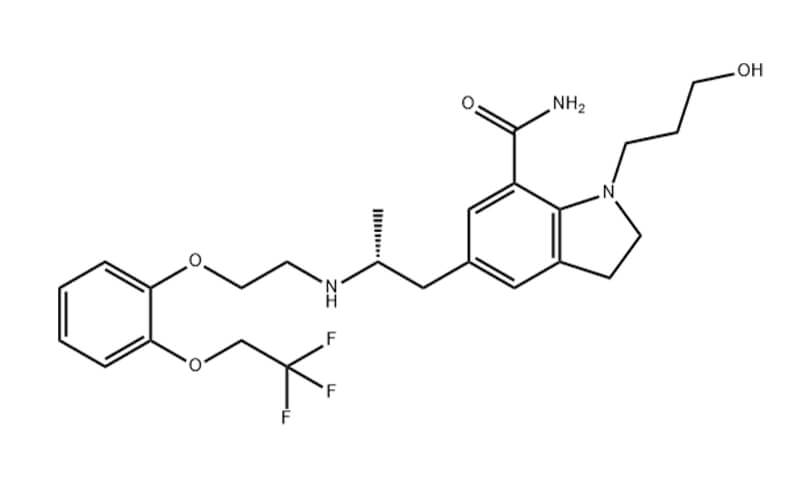
Lenvatinib API: A Potential Treatment for Thyroid Cancer
Lenvatinib , a multikinase inhibitor, has emerged as a promising therapeutic option for the treatment of differentiated thyroid cancer (DTC), particularly in patients with radioiodine-refractory disease (RRDTC). This article delves into the pharmacological properties, clinical efficacy, and safety profile of lenvatinib, highlighting its potential as a valuable treatment for DTC.
What is Differentiated Thyroid Cancer(DTC)?
Differentiated thyroid cancer (DTC) is the most common type of thyroid cancer, accounting for over 90% of all cases. It is a slow-growing cancer that typically arises from the follicular cells of the thyroid gland. DTC has a very good prognosis, with a 10-year survival rate of over 98%.
There are two main types of DTC:
- Papillary thyroid cancer: This is the most common type of DTC, accounting for about 80% of all cases. It is characterized by its papillary growth pattern, which means that the cancer cells form finger-like projections.
- Follicular thyroid cancer: This is the second most common type of DTC, accounting for about 10-15% of all cases. It is characterized by its follicular growth pattern, which means that the cancer cells form small follicles.
Symptoms of DTC
Potential symptoms of DTC may include:
- A painless lump in the neck: This is the most common symptom, often felt near the Adam’s apple.
- Changes in voice or hoarseness: This can occur if the tumor presses on the vocal cords.
- Swollen lymph nodes in the neck: This can happen if the cancer has spread.
- Difficulty swallowing: This can occur if the tumor is large enough to press on the esophagus.
- Neck or facial pain: This is less common but can occur if nerves or muscles are involved.
However, it is important to remember that these symptoms can be caused by other conditions, and their presence does not necessarily indicate DTC.
Lenvatinib API Mechanism of Action
Lenvatinib API is a multikinase inhibitor that inhibits the vascular endothelial growth factor (VEGF) receptors VEGFR1, VEGFR2, and VEGFR3. It also inhibits the fibroblast growth factor receptors FGFR1, FGFR2, FGFR3, and FGFR4, as well as the platelet-derived growth factor receptor PDGFRα. By inhibiting these receptors, lenvatinib blocks the signaling pathways that are involved in tumor angiogenesis, tumor growth, and tumor progression. This makes lenvatinib an effective treatment for a variety of cancers, including thyroid cancer, liver cancer, and renal cell carcinoma.
Here is a more detailed explanation of the mechanism of action of lenvatinib API:
- VEGF receptors: VEGF is a protein that is involved in the formation of new blood vessels. By inhibiting VEGFR1, VEGFR2, and VEGFR3, lenvatinib blocks the formation of new blood vessels in tumors, which can starve the tumors of oxygen and nutrients and prevent them from growing.
- FGFR receptors: FGFRs are a family of proteins that are involved in cell growth, proliferation, and differentiation. By inhibiting FGFR1, FGFR2, FGFR3, and FGFR4, lenvatinib blocks the growth and proliferation of tumor cells.
- PDGFRα: PDGFRα is a protein that is involved in cell proliferation and migration. By inhibiting PDGFRα, lenvatinib blocks the migration of tumor cells to other parts of the body.
Lenvatinib is an effective treatment for a variety of cancers, and it is generally well-tolerated. However, it can cause some side effects, such as diarrhea, fatigue, and hypertension.
Clinical Efficacy of Lenvatinib API
Lenvatinib API has demonstrated clinical efficacy in the treatment of several types of cancer, including:
- Thyroid cancer: Lenvatinib is approved by the U.S. Food and Drug Administration (FDA) for the treatment of differentiated thyroid cancer (DTC) that is locally advanced or unresectable, and that is resistant to radioactive iodine therapy. In clinical trials, lenvatinib has shown significant improvement in progression-free survival (PFS) and overall survival (OS) compared to placebo or sorafenib.
- Liver cancer: Lenvatinib is approved by the FDA for the treatment of hepatocellular carcinoma (HCC) in patients who have previously been treated with sorafenib. In clinical trials, lenvatinib has shown significant improvement in PFS and OS compared to placebo.
- Renal cell carcinoma: Lenvatinib is approved by the FDA for the treatment of advanced renal cell carcinoma (RCC) in patients who have previously been treated with an inhibitor of vascular endothelial growth factor (VEGF) and an inhibitor of mammalian target of rapamycin (mTOR). In clinical trials, lenvatinib has shown significant improvement in PFS and OS compared to everolimus.
Potential Role of Lenvatinib API in the Treatment of DTC
Lenvatinib API has demonstrated significant clinical efficacy in the treatment of differentiated thyroid cancer (DTC), particularly in patients with radioactive iodine-refractory (RAI) DTC. Here’s a summary of its potential role in DTC treatment:
- RAI-refractory DTC: Lenvatinib has emerged as a valuable treatment option for patients with RAI-refractory DTC, a subset of the disease that is resistant to radioactive iodine therapy, the standard first-line treatment for DTC. Clinical trials have consistently shown that lenvatinib improves progression-free survival (PFS) and overall survival (OS) compared to placebo or other standard-of-care therapies in patients with RAI-refractory DTC.
- First-line treatment for advanced DTC: While lenvatinib is primarily used for RAI-refractory DTC, there is growing evidence suggesting its potential role as a first-line treatment option for advanced DTC, especially in patients with aggressive or high-risk disease. Studies have shown that lenvatinib, either alone or in combination with other targeted therapies, can achieve favorable PFS and OS rates in this patient population.
- Combination therapy: Lenvatinib has also been investigated in combination with other targeted therapies, such as sorafenib and everolimus, for the treatment of DTC. These combinations have shown promising results in terms of improved PFS and OS compared to monotherapy with either lenvatinib or the other agent alone.
Potential Benefits of Lenvatinib
Lenvatinib offers several potential benefits over other treatment options for DTC:
- Durable response: Studies have shown that lenvatinib can induce durable responses in patients with DTC, meaning that patients can maintain disease control for extended periods.
- Favorable safety profile: Lenvatinib is generally well-tolerated, with a manageable side effect profile compared to other targeted therapies.
Summary
Overall, Lenvatinib API plays a significant role in the pharmaceutical industry as an effective and well-tolerated treatment for various types of cancer, offering substantial benefits to patients with these challenging diseases. Ongoing research continues to explore the potential of lenvatinib in combination with other therapies to further improve treatment outcomes.
Qingmu Pharmaceutical is a leading Lenvatinib manufacturer in China. The company has a strong track record of supplying high-quality lenvatinib API to pharmaceutical companies around the world. Qingmu Pharmaceutical is committed to providing its customers with reliable and affordable lenvatinib API.