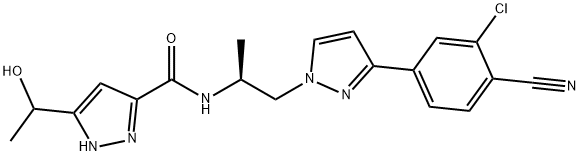
Lenvatinib Mesilate API
| CAS No. | 857890-39-2 |
| Therapeutic Category | Anti-Cancer/ Oncology |
| Technology | Synthetic |
| Dosage Form | Oral Solids |
| Innovator Brand | Lenvima(USA) |
| Registration Status | China: I Others: DMF |
| Polymorph | Form C; New form with own patent |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description of Lenvatinib Mesilate API
- Grade: Active Pharmaceutical Ingredient(API)
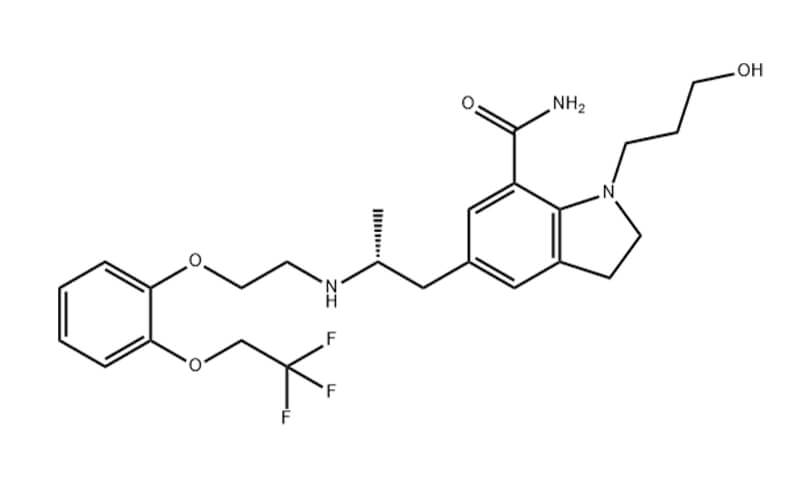
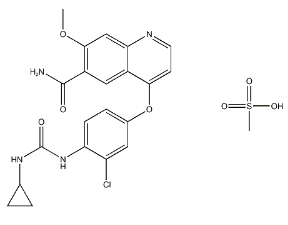
- Chemical Name: 4-{3-Chloro-4-[(cyclopropylcarbamoyl)amino]phenoxy}-7-methoxy-6-quinolinecarboxamide
- Molecular Formula: C22H23ClN4O7S
- Molecular Weight: 522.96
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Keep in an airtight container and store at 2~8 centigrade, protected from light.
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag or according to customer’s requirement.
Applications of Lenvatinib Mesilate API
- Lenvatinib API is a receptor tyrosine kinase (RTK) inhibitor that inhibits the kinase activities of vascular endothelial growth factor (VEGF) receptors VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). Lenvatinib also inhibits other RTKs that have been implicated in pathogenic angiogenesis, tumor growth, and cancer progression in addition to their normal cellular functions, including fibroblast growth factor (FGF) receptors FGFR1, 2, 3, and 4; the platelet-derived growth factor receptor alpha (PDGFRα), KIT, and RET. These receptor tyrosine kinases (RTKs) located in the cell membrane play a central role in the activation of signal transduction pathways involved in the normal regulation of cellular processes, such as cell proliferation, migration, apoptosis and differentiation, and in pathogenic angiogenesis, lymphogenesis, tumor growth, and cancer progression. In particular, VEGF has been identified as a crucial regulator of both physiologic and pathologic angiogenesis and increased expression of VEGF is associated with a poor prognosis in many types of cancers.
- Lenvatinib API is indicated for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine (RAI)-refractory differentiated thyroid cancer. Most patients with thyroid cancer have a very good prognosis with treatment (98% 5-year survival rate) involving surgery and hormone therapy. However, for patients with RAI-refractory thyroid cancer, treatment options are limited and the prognosis is poor, leading to a push for the development of more targeted therapies such as lenvatinib.
Why Choose Us as Your Lenvatinib Factory?
- Qigmu is a Lenvatinib manufacturer, our Lenvatinib API has been approved in China(DMF filed and listed on CDE’s website), DMF in CTD format is available and can be supported for registration worldwide.
- Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, no risk to remove factory. A new API factory is under design and is predicted to be put into use in 2025.
- As a Lenvatinib factory, our team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. We passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) and also customer audits from Europe, USA, Japan, etc.
- Qingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil. You can rest assured to choose our products.