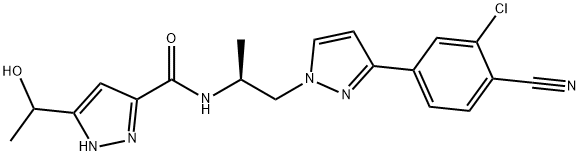
Aprepitant API
| CAS No. | 170729-80-3 |
| Therapeutic Category | Anti-Cancer/ Oncology |
| Technology | Synthetic |
| Dosage Form | Oral Solids |
| Innovator Brand | Emend(USA) |
| Registration Status | China: I EU: CEP2021-323 Others: DMF |
| Polymorph | Form I |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
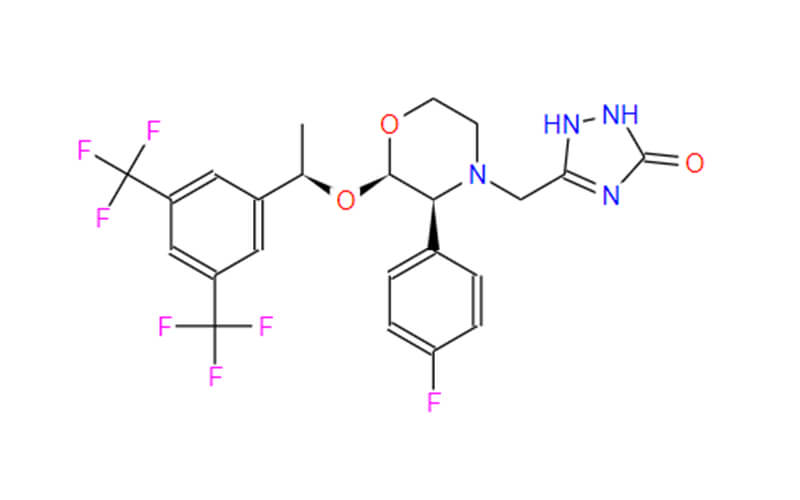
- Chemical Name: 5-{[(2R,3S)-2-{(1R)-1-[3,5-Bis(trifluormethyl)phenyl]ethoxy}-3-(4-fluorphenyl)morpholin-4-yl]methyl}-1,2-dihydro-3H-1,2,4-triazol-3-on
- Molecular Formula: C23H21F7N4O3
- Molecular Weight: 534.427
- Specification: EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Aprepitant
- Aprepitant is an antiemetic chemical compound that belongs to “substance P” antagonists (SPA) with its effect being blocking the neurokinin 1(Nk1) receptor. It is used for the prevention of acute and delayed chemotherapy-induced nausea and vomiting(CINV) and for the prevention of postoperative nausea and vomiting. It can also be used for the treatment of cyclic vomiting syndrome and late-stage chemotherapy-induced vomiting occurring during cancer treatment.
- Aprepitant alleviates the case of vomiting in patients through balking the signals released by Nk1 receptors. Nk1 is a G-protein-coupled receptor with its ligand being substance P (SP). The high concentration of SP is required for the vomiting reflex. Aprepitant blocks the process of SP-NK1 signaling in activating the vomiting reflex.
Why Choose Us as Your Manufacturer?
- As an Aprepitant manufacturer, Qingmu‘s Aprepitant has been approved in China(DMF filed and listed on CDE’s website) and also got the CEP certificate in EDQM, DMF in CTD format is available and can be supported for registration worldwide.
- Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, no risk to remove factory. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. We passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) and also customer audits from Europe, USA and Japan, etc. So please feel free to choose our products.
- Qiingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.