Nilotinib Hydrochloride: A Targeted Therapy for Chronic Myelogenous Leukemia
Chronic myelogenous leukemia (CML) is a type of blood cancer characterized by the uncontrolled growth of white blood cells in the bone marrow. This abnormal growth stems from a genetic mutation known as the Philadelphia chromosome, where parts of chromosomes 9 and 22 fuse to create a new chromosome. This fusion creates the Bcr-Abl protein, which fuels the uncontrolled proliferation of leukemia cells.
Nilotinib hydrochloride is a medication specifically designed to combat CML. This article delves into the properties, applications, and considerations surrounding nilotinib hydrochloride, particularly its role as an active pharmaceutical ingredient (API).
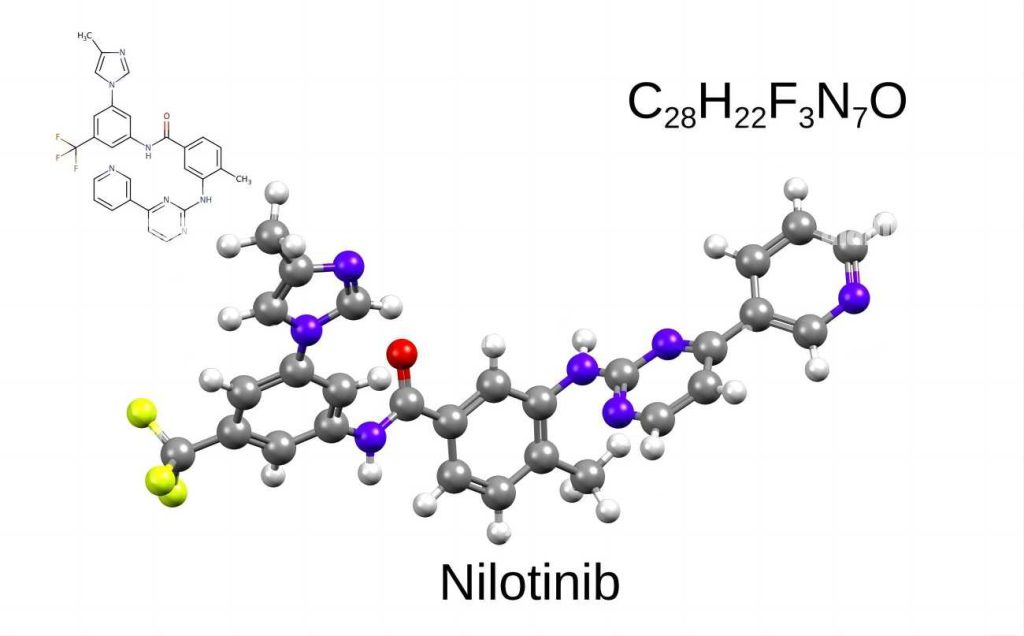
What is Nilotinib Hydrochloride?
1. Chemical Composition and Formula
Nilotinib hydrochloride, also known by the identifier AMN-107 HCl, is a specific chemical compound derived from nilotinib. It exists in a salt form where nilotinib interacts with hydrochloric acid (HCl). This interaction adds a chlorine atom (Cl) and a hydrogen atom (H) to the nilotinib molecule, altering its overall electrical properties.
The precise structure of nilotinib hydrochloride can be represented by its chemical formula, C28H23ClF3N7O. This formula breaks down the molecule into its constituent atoms:
- 28 Carbon (C) atoms
- 23 Hydrogen (H) atoms
- 1 Chlorine (Cl) atom (introduced by the hydrochloric acid)
- 3 Fluorine (F) atoms
- 7 Nitrogen (N) atoms
- 2 Oxygen (O) atoms
These atoms are arranged in a specific three-dimensional configuration that determines the molecule’s properties and biological activity.
2. Properties of Nilotinib Hydrochloride
Nilotinib hydrochloride appears as a white to slightly yellow crystalline powder. Its solubility in water is limited, meaning it only dissolves to a small extent in water. This characteristic can be crucial for formulating the medication into a usable dosage form, such as tablets.
Another important physical property is its melting point, which is a well-defined temperature at which the solid powder transitions into a liquid state. Maintaining specific storage conditions is essential to ensure the nilotinib hydrochloride remains stable and retains its potency.
3. Nilotinib Hydrochloride vs. Nilotinib Hydrochloride Monohydrate
It’s important to differentiate between nilotinib hydrochloride and its close relative, nilotinib hydrochloride monohydrate. The key difference lies in the presence of water molecules. Nilotinib hydrochloride monohydrate has one water molecule (H2O) bonded to each individual molecule of nilotinib hydrochloride. This additional water content can slightly alter physical properties like melting point and solubility compared to the anhydrous form (without water) of nilotinib hydrochloride.
However, despite this difference in water content, both nilotinib hydrochloride and its monohydrate form possess the same therapeutic activity against chronic myelogenous leukemia (CML). This means they have the same effectiveness in targeting and treating the disease.
Nilotinib Hydrochloride as an Active Pharmaceutical Ingredient (API)
The Role of an API in Medication
In any pharmaceutical product, the active pharmaceutical ingredient (API) plays a central role. It’s the specific molecule designed to produce the desired therapeutic effect within the body. Essentially, the API interacts with the body’s biological processes in a targeted way to treat or prevent a disease. In the case of nilotinib hydrochloride, it serves as the critical API in medications formulated to combat chronic myelogenous leukemia (CML).
Applications of Nilotinib Hydrochloride API
The primary application of nilotinib hydrochloride API lies in the development of tablets for oral administration. These tablets target CML, specifically cases with the Philadelphia chromosome mutation. Here’s a closer look at how nilotinib hydrochloride API is used in CML treatment:
- Chronic Phase CML: Nilotinib can be used as the initial treatment (first-line therapy) for patients newly diagnosed with chronic phase CML. Additionally, it can be used for patients who experience intolerance or show no response to imatinib, another medication used for CML treatment.
- Accelerated Phase CML: When CML progresses to the accelerated phase, characterized by a rapid increase in white blood cell production, nilotinib can be used to slow this progression and potentially return the disease to the chronic phase.
- Blast Crisis CML: The most advanced stage of CML is blast crisis, where leukemia cells transform into immature blasts. While nilotinib is not the primary treatment for this stage, it may be incorporated into specific treatment regimens.
It’s important to note that the use of nilotinib hydrochloride API in medications is determined by physicians based on the specific stage and characteristics of a patient’s CML.
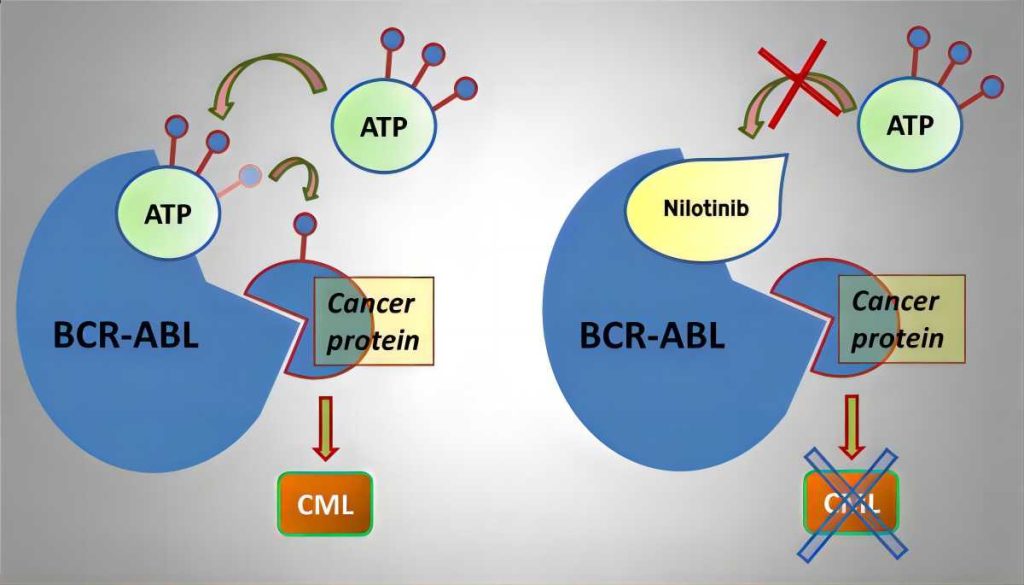
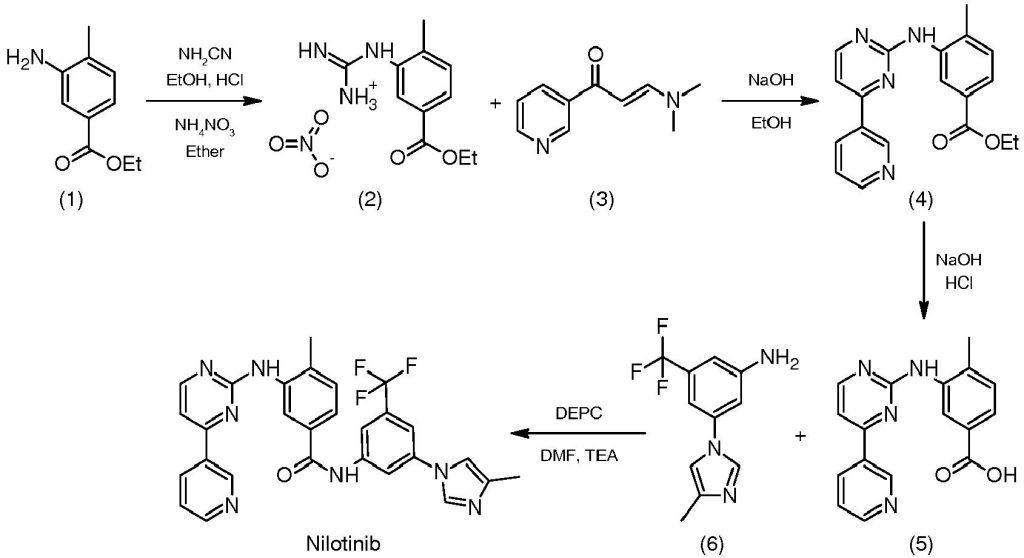
Manufacturing of Nilotinib Hydrochloride API
The manufacturing process of nilotinib hydrochloride API involves a series of complex chemical reactions. Due to the intricate nature of the process and the need for stringent quality control, the specifics are typically considered proprietary information by pharmaceutical companies. However, the general steps can involve:
- Obtaining or Synthesizing Starting Materials: The manufacturing journey begins with acquiring or synthesizing simpler chemical compounds that will serve as the building blocks for nilotinib. These starting materials possess less complex chemical structures compared to the final product.
- Controlled Chemical Reactions: The obtained starting materials are then subjected to a meticulously controlled series of chemical reactions. These reactions involve precise manipulation of conditions like temperature, pressure, and reaction time to ensure the efficient and specific formation of nilotinib.
- Rigorous Purification: Following the initial chemical reactions, the resulting product, which is a crude form of nilotinib, undergoes a series of rigorous purification processes. These processes are designed to meticulously remove any impurities that may be present. Achieving a high degree of purity for the API is essential for its safety and efficacy in the final medication.
- Crystallization and Drying: Once the purification stage is complete, the purified nilotinib is subjected to a process called crystallization. Crystallization allows for obtaining the desired solid form of the API with specific physical characteristics. After crystallization, the nilotinib is then dried to eliminate any residual solvents that may have been used during the manufacturing process.
- Extensive Quality Control Testing: The final step involves putting the manufactured nilotinib hydrochloride API through a battery of quality control tests. These tests meticulously assess the API against predetermined specifications for critical parameters like purity, potency, and other relevant characteristics. Only after passing these stringent quality control measures can the nilotinib hydrochloride API be deemed suitable for use in pharmaceutical formulations.
It’s important to remember that this is a simplified overview of the general steps involved. The actual manufacturing process is likely to be far more intricate and involve additional stages to ensure consistent quality and safety of the final API.
Considerations for Nilotinib Hydrochloride
Safety and Side Effects
While nilotinib hydrochloride is an effective treatment for CML, it can cause side effects. Some common side effects include rash, fatigue, headache, diarrhea, and nausea. More serious side effects, though less frequent, can occur and require immediate medical attention. These include fluid buildup around the lungs (pleural effusion), low blood cell counts, and liver problems.
It’s crucial for patients taking nilotinib hydrochloride to be closely monitored by their doctor throughout treatment. This monitoring includes:
- Regular Blood Tests: Frequent blood tests are essential to assess complete blood count (CBC) and monitor for potential side effects like low white blood cell counts, which can increase the risk of infection.
- Liver Function Tests: Since nilotinib can affect the liver, regular liver function tests are necessary to detect any abnormalities.
- Electrolyte Monitoring: Electrolyte imbalances, particularly low levels of potassium or magnesium, can occur with nilotinib use. These imbalances require monitoring and correction if necessary.
- Pregnancy Testing: Nilotinib can cause severe birth defects. Women of childbearing age should use effective contraception during treatment and for a specific period after discontinuation. Men taking nilotinib should also use contraception if planning to conceive a child with a partner.
Regulations and Approvals
Nilotinib hydrochloride has undergone rigorous clinical trials to evaluate its safety and efficacy. Following successful trials, it received approval from regulatory agencies like the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) for the treatment of CML. These approvals ensure that the medication meets specific safety and effectiveness standards.
Conclusion
Nilotinib hydrochloride is a valuable tool in the fight against chronic myelogenous leukemia. As an API, it forms the core of medications that target the Bcr-Abl protein, a key driver of CML. Understanding the properties, applications, and considerations surrounding nilotinib hydrochloride is essential for both healthcare professionals and patients. With continued research and development, nilotinib and similar targeted therapies hold promise for improving the lives of CML patients.

