Apalutamide API vs. Abiraterone Acetate: A Comparative Analysis
When grappling with castration-resistant prostate cancer (CRPC), understanding treatment options is critical. Apalutamide API and Abiraterone Acetate are two prominent medications that target the androgen receptor (AR) pathway, a key driver of prostate cancer growth. This article delves into a comparative analysis of these medications, helping you grasp their individual functionalities and how they stack up against each other.
Understanding Apalutamide API
Apalutamide API, a groundbreaking development in oncology, operates at the forefront of precision medicine against prostate cancer. Its mechanism of action revolves around selective inhibition of the androgen receptor (AR), a pivotal target in the progression of prostate cancer. By specifically targeting the AR, Apalutamide disrupts the signaling cascade driven by androgens, effectively hindering the growth and proliferation of prostate cancer cells. This inhibition is particularly crucial in the context of non-metastatic castration-resistant prostate cancer (nmCRPC), where the disease exhibits resistance to conventional androgen deprivation therapy.
The approval of Apalutamide by the FDA in 2018 marked a significant milestone, offering a ray of hope for patients facing the challenges of nmCRPC. Clinical trials have underscored its efficacy in delaying disease progression and improving patient outcomes, setting a new standard of care in the management of this aggressive form of prostate cancer. Moreover, its favorable safety profile further enhances its appeal, providing patients with a therapeutic option that balances efficacy with tolerability. Apalutamide API not only represents a triumph in scientific innovation but also embodies a beacon of hope for patients and healthcare providers alike, driving advancements in personalized cancer treatment.
Exploring Abiraterone Acetate
Abiraterone Acetate, a formidable contender in the realm of prostate cancer treatment, operates through a multifaceted mechanism that targets the very source of androgen production. By inhibiting the enzyme CYP17, Abiraterone disrupts the synthesis of androgens not only from the testes but also from adrenal glands and prostate tumor tissues. This comprehensive blockade of androgen biosynthesis effectively deprives prostate cancer cells of the fuel they need to proliferate, leading to a significant reduction in tumor burden and disease progression. Moreover, Abiraterone’s efficacy extends beyond the confines of traditional androgen deprivation therapy, offering a promising avenue for patients with metastatic castration-resistant prostate cancer (mCRPC) who have experienced disease progression despite prior treatments. Its approval in 2011 marked a pivotal moment in oncology, providing a much-needed therapeutic option that has since become a cornerstone in the management of advanced prostate cancer.
Comparative Analysis between Apalutamide API and Abiraterone Acetate
1. Mechanism of Action:
- Apalutamide API: Acts as a selective inhibitor of the androgen receptor (AR), preventing androgens from binding to the AR and subsequently inhibiting AR-mediated signaling pathways. By targeting the androgen receptor, Apalutamide disrupts the growth and proliferation of prostate cancer cells.
- Abiraterone Acetate: Functions as a potent CYP17 inhibitor, blocking the enzyme responsible for androgen biosynthesis in the adrenal glands and tumor tissues. By inhibiting CYP17, Abiraterone reduces the production of androgens, thereby depriving prostate cancer cells of the hormones necessary for their growth and survival.
2. Indications:
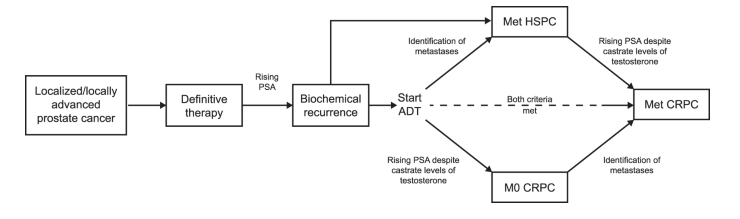
- Apalutamide API: Primarily indicated for the treatment of non-metastatic castration-resistant prostate cancer (nmCRPC), a stage of prostate cancer where the disease progresses despite castrate levels of testosterone.
- Abiraterone Acetate: Indicated for the management of metastatic castration-resistant prostate cancer (mCRPC), a more advanced stage of prostate cancer characterized by disease spread beyond the prostate gland and resistance to hormonal therapies.
3. Efficacy:
- Clinical trials evaluating the efficacy of Apalutamide API have demonstrated its ability to significantly prolong metastasis-free survival (MFS) and delay the onset of symptomatic progression in patients with nmCRPC. Additionally, Apalutamide has shown benefits in terms of delaying the need for subsequent anticancer therapies.
- Abiraterone Acetate has exhibited notable efficacy in improving overall survival, radiographic progression-free survival (rPFS), and quality of life in patients with mCRPC. It has also demonstrated efficacy in both chemotherapy-naive and post-chemotherapy settings.
4. Side Effects:
- Apalutamide API: Common side effects associated with Apalutamide include fatigue, hypertension, rash, diarrhea, and musculoskeletal events such as fractures and falls. Additionally, Apalutamide may lead to hypothyroidism and elevations in liver enzymes.
- Abiraterone Acetate: Adverse reactions commonly observed with Abiraterone include mineralocorticoid excess (manifesting as fluid retention, edema, hypokalemia), hepatotoxicity, hypertension, and cardiac disorders. Patients may also experience gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
5. Drug Interactions:
Both Apalutamide and Abiraterone can interact with other medications, necessitating careful consideration of concomitant use. Apalutamide is a strong inducer of CYP3A4 and may decrease the plasma concentrations of drugs metabolized by this enzyme. Abiraterone is primarily metabolized by CYP3A4, and co-administration with strong CYP3A4 inhibitors or inducers may alter its pharmacokinetics.
In conclusion, while Apalutamide API and Abiraterone Acetate share the common goal of combating prostate cancer, they differ in their mechanisms of action, indications, efficacy profiles, and side effect profiles. The choice between Apalutamide and Abiraterone depends on various factors, including the stage of prostate cancer, disease characteristics, patient preferences, and comorbidities. Healthcare providers should carefully weigh these considerations to tailor treatment decisions to individual patient needs, ultimately optimizing therapeutic outcomes and improving quality of life for patients with prostate cancer.
Conclusion
Apalutamide API and Abiraterone Acetate represent valuable tools with distinct advantages and considerations in the fight against CRPC. Their mechanisms of action offer different benefits and drawbacks in terms of efficacy and side effects. Consulting a healthcare professional is essential to determine the most appropriate treatment course based on the specifics of your case. By understanding these medications and their nuances, you can actively participate in informed discussions with your doctor about the best course of action for managing CRPC and achieving the best possible treatment outcomes.