Similarities and Differences Between Ruxolitinib Phosphate and Ruxolitinib API
The relentless pursuit of novel medications is a cornerstone of progress in the medical field. In recent years, Ruxolitinib Phosphate and Ruxolitinib API have emerged as promising therapeutic agents, garnering significant interest for their potential in treating specific ailments. However, a clear understanding of the distinctions and interrelationships between these two entities remains crucial for optimal utilization. This article delves into the complexities of Ruxolitinib Phosphate and Ruxolitinib API, exploring their chemical structures, manufacturing processes, pharmacological effects, and clinical applications.
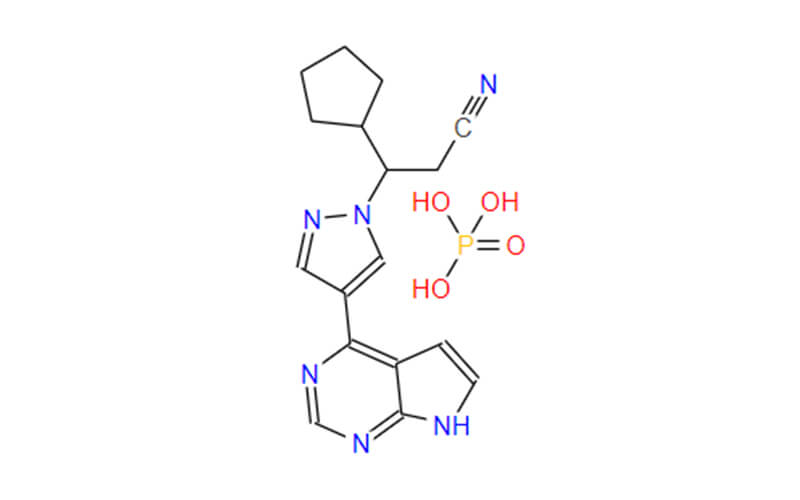
Fundamental Differences: Ruxolitinib Phosphate vs. Ruxolitinib API
- Ruxolitinib Phosphate: This drug represents a novel small-molecule tyrosine kinase inhibitor (TKI). TKIs exert their anti-tumor effects by specifically targeting and inhibiting tyrosine kinases, enzymes that play a crucial role in promoting tumor cell growth and proliferation. Notably, Ruxolitinib Phosphate functions as a prodrug, a medication that undergoes transformation within the body to release its active form. In this case, Ruxolitinib Phosphate rapidly converts to its active metabolite, Ruxolitinib, upon entering the body.
- Ruxolitinib API (Active Pharmaceutical Ingredient): This term refers to the core active component utilized in the production of Ruxolitinib and its derivatives. Unlike Ruxolitinib Phosphate, Ruxolitinib API exists in its active form and is typically available as a powder. It can be further processed and formulated into various drug delivery systems for clinical use.
While both entities belong to the same class of medications, key distinctions exist:
- Chemical Structure: Ruxolitinib Phosphate boasts a more complex chemical structure compared to Ruxolitinib API. This complexity arises from the presence of a phosphate ester group attached to Ruxolitinib Phosphate’s core molecule. This difference can influence their physical and chemical properties.
- Pharmacological Effects: Due to the prodrug nature of Ruxolitinib Phosphate, its anti-tumor activity might exhibit slight variations compared to Ruxolitinib API. However, both are expected to possess similar mechanisms of action, primarily targeting and inhibiting specific tyrosine kinases within tumor cells.
- Clinical Applications: Ruxolitinib Phosphate has undergone rigorous clinical trials and received regulatory approval for the treatment of specific cancers, including non-small cell lung cancer (NSCLC) and kidney cancer. It is typically administered orally, either as a single agent or in combination with other therapies. Clinical studies have demonstrated its efficacy and a generally acceptable safety profile.
On the other hand, Ruxolitinib API currently holds limited application in clinical settings. Its primary function lies within research and development, serving as the foundation for the production of Ruxolitinib Phosphate and potentially other Ruxolitinib-based medications. However, ongoing research into Ruxolitinib’s mechanisms of action and its potential for broader clinical use is promising.

Ruxolitinib Phosphate vs. API: Structure and Synthesis
1. Chemical Structure:
- Ruxolitinib Phosphate: This molecule possesses a complex structure featuring multiple functional groups. At its core lies a tetracyclic structure, a ring system composed of four interconnected carbon rings. This core structure incorporates several nitrogen and carbon atoms. Additionally, a phosphate ester group is attached to a benzene ring within the molecule. The intricate nature of this structure contributes to Ruxolitinib Phosphate’s unique physical and chemical properties.
- Ruxolitinib API: Compared to Ruxolitinib Phosphate, the structure of Ruxolitinib API is considerably simpler. It revolves around a five-membered heterocyclic ring, a ring structure containing both carbon and another element, typically nitrogen. A benzene ring is also present within the molecule, along with nitrogen and carbon atoms. However, the crucial distinction lies in the absence of the phosphate ester group found in Ruxolitinib Phosphate. This structural difference translates to distinct physical and chemical properties for Ruxolitinib API.
2. Synthesis Methods:
Ruxolitinib Phosphate: The synthesis of Ruxolitinib Phosphate involves a multi-step chemical process. This process typically includes:
- Cyclization reactions: These reactions are responsible for constructing the core tetracyclic structure of the molecule.
- Substitution reactions: These reactions introduce nitrogen and carbon atoms into specific positions within the molecule.
- Esterification reactions: The final step involves the attachment of the phosphate ester group to the designated location on the molecule.
Ruxolitinib API: The synthesis of Ruxolitinib API is generally less complex compared to Ruxolitinib Phosphate. It primarily involves:
- Cyclization reactions: Similar to Ruxolitinib Phosphate, these reactions form the core five-membered heterocyclic ring.
- Substitution reactions: These reactions introduce nitrogen and carbon atoms into specific positions within the Ruxolitinib API molecule.
- Purification and drying: Following the completion of the reaction sequence, the product undergoes rigorous purification processes to eliminate impurities and ensure the desired level of purity for Ruxolitinib API. This step often involves techniques like filtration, crystallization, and drying under controlled conditions. Maintaining optimal reaction temperature, time, and pressure throughout the synthesis process is crucial for both Ruxolitinib Phosphate and Ruxolitinib API to guarantee high yield and purity of the final product.
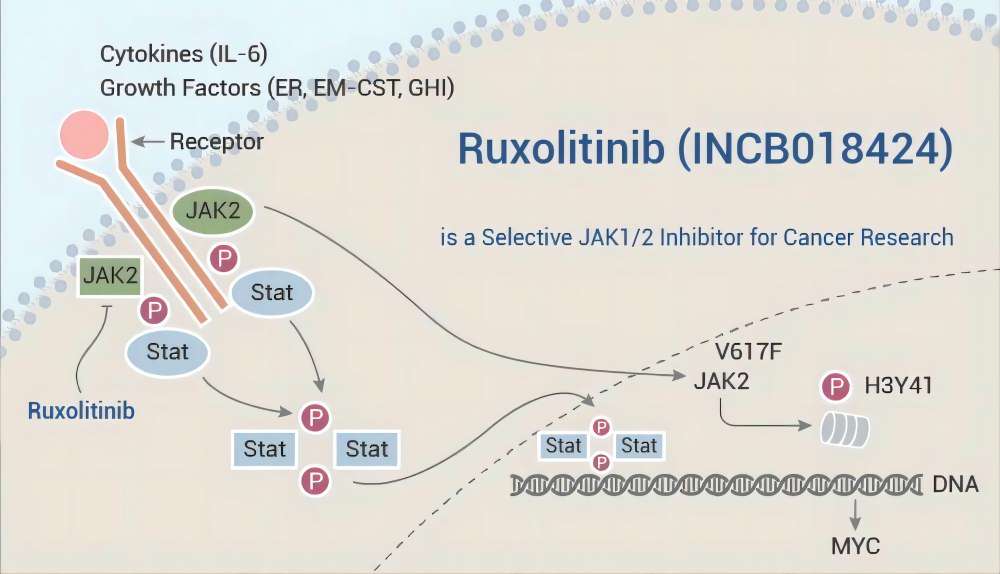
Mechanisms of Action and Therapeutic Effects
As previously mentioned, both Ruxolitinib Phosphate and Ruxolitinib API function as tyrosine kinase inhibitors. Tyrosine kinases are enzymes responsible for phosphorylating specific proteins within cells. This phosphorylation process plays a critical role in regulating various cellular functions, including cell growth, proliferation, and survival. In the context of cancer, abnormal activation of tyrosine kinases can contribute to uncontrolled tumor cell growth and proliferation.
Ruxolitinib Phosphate and Ruxolitinib API specifically target and inhibit particular Janus kinase (JAK) enzymes, a sub-class of tyrosine kinases. JAKs are involved in cytokine signaling pathways, which are essential for communication between cells. By inhibiting JAK enzymes, these drugs can disrupt the uncontrolled growth and survival signals received by tumor cells. This disruption ultimately leads to the inhibition of tumor cell proliferation and can even induce tumor cell death (apoptosis).
It is important to note that while both Ruxolitinib Phosphate and Ruxolitinib API target JAK enzymes, there might be slight variations in their specific target profiles or potency. Ongoing research is actively investigating these potential differences to optimize therapeutic strategies.
Clinical Applications and Safety Considerations
As previously discussed, Ruxolitinib Phosphate has established its place in clinical oncology for treating specific cancers. Its efficacy and safety profile have been validated through clinical trials, leading to regulatory approval for conditions like NSCLC and kidney cancer. It is typically administered orally, offering a convenient route of administration for patients. Ruxolitinib Phosphate can be used as a monotherapy (single agent) or combined with other chemotherapeutic drugs for a more comprehensive treatment approach. Clinical studies have demonstrated promising results with Ruxolitinib Phosphate, including improved patient outcomes and a generally well-tolerated safety profile. However, as with any medication, potential side effects can occur. Common side effects associated with Ruxolitinib Phosphate include anemia (low red blood cell count), thrombocytopenia (low platelet count), and fatigue. Careful monitoring by healthcare professionals is essential to manage these potential side effects and ensure patient safety.
Ruxolitinib API, on the other hand, is not currently used directly in clinical settings. Its primary application lies in the research and development of Ruxolitinib Phosphate and potentially other Ruxolitinib-based medications. However, the ongoing research into Ruxolitinib’s mechanisms of action and its potential for broader therapeutic use is encouraging. Future studies might explore the possibility of utilizing Ruxolitinib API in specific drug delivery systems or formulations to enhance its therapeutic potential.
Conclusion
Ruxolitinib Phosphate and Ruxolitinib API, while sharing the core name “Ruxolitinib,” represent distinct entities with crucial roles in cancer treatment. Ruxolitinib Phosphate, as a clinically approved drug, offers a valuable tool for oncologists in the fight against specific malignancies. Ruxolitinib API, on the other hand, serves as the foundation for Ruxolitinib Phosphate production and holds promise for future therapeutic advancements. As research continues to delve deeper into the mechanisms of action and potential applications of Ruxolitinib, we can anticipate the development of more targeted and effective treatment strategies for various cancers. This ongoing exploration paves the way for a future with more options for patients battling cancer and a brighter outlook for improved patient outcomes.
If you are looking for Ruxolitinib manufacturer for business cooperation, Qingmu is a good choice to you.