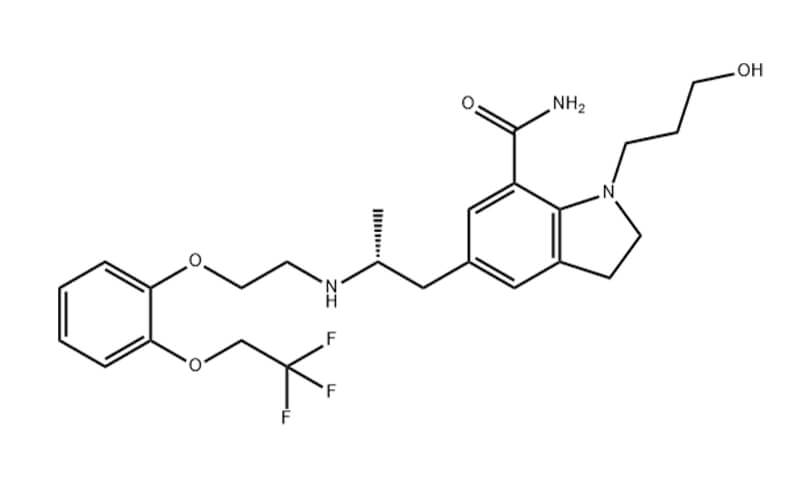
Relugolix API
| CAS No. | 737789-87-6 |
| Therapeutic Category | Anti-Cancer/ Oncology |
| Technology | Synthetic |
| Dosage Form | Oral Solids |
| Innovator Brand | Myfembree, Orgovyx(USA) |
| Registration Status | DMF under preparation |
| Polymorph | N/A |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
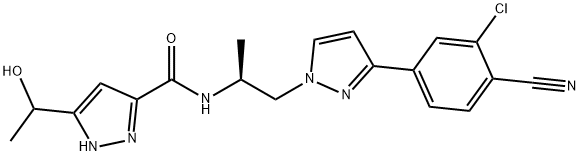
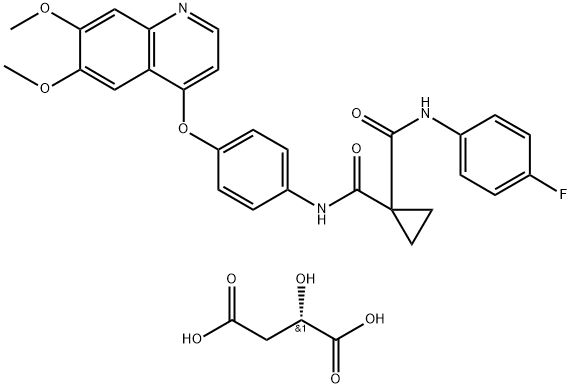
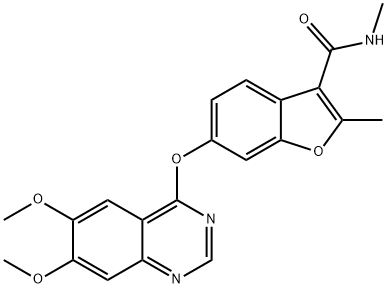
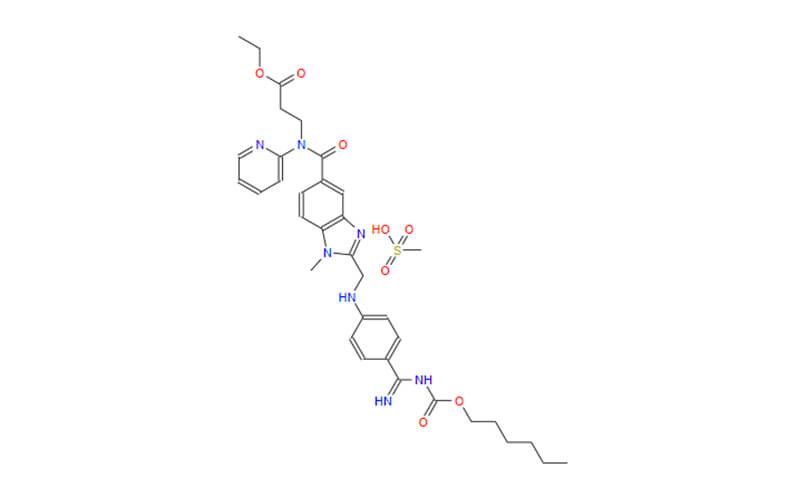
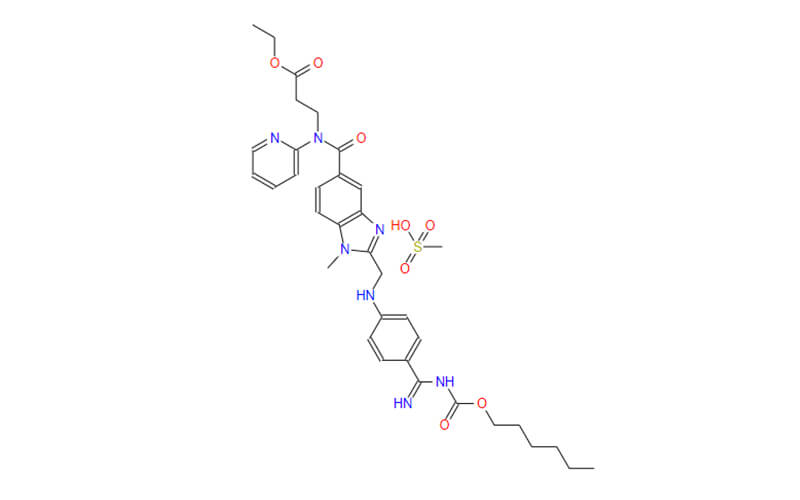
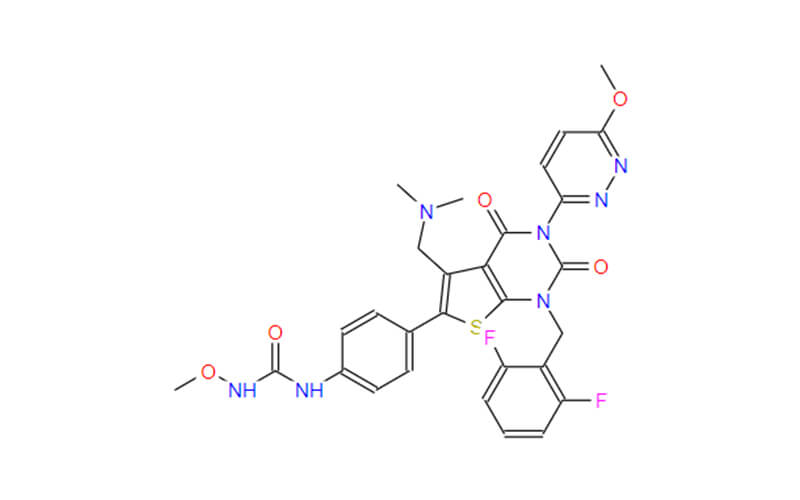
- Chemical Name:1-[4-[1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxothieno[2,3-d]pyrimidin-6-yl]phenyl]-3-methoxyurea
- Molecular Formula: C29H27F2N7O5S
- Molecular Weight: 623.63
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Relugolix API
- Relugolix API is a gonadotropin-releasing hormone (GnRH) receptor antagonist used in the treatment of several hormone-responsive conditions. It was first approved in Japan in 2019, under the brand name Relumina, for the symptomatic treatment of uterine fibroids, and more recently by the United States FDA in 2020, under the brand name Orgovyx, for the treatment of advanced prostate cancer. This branded product was later approved by the European Commission on April 29, 2022. Relugolix has also been studied in the symptomatic treatment of endometriosis.
- Relugolix API is the first (and currently only) orally-administered GnRH receptor antagonist approved for the treatment of prostate cancer – similar therapies such as degarelix require subcutaneous administration – and therefore provides a less burdensome therapeutic option for patients who might otherwise require clinic visits for administration by healthcare professionals. In addition to its relative ease of use, relugolix was shown to be superior in the depression of testosterone levels when compared to leuprolide, another androgen deprivation therapy used in the treatment of prostate cancer. In May 2021, the FDA approved the combination product made up of relugolix, estradiol, and norethindrone under the market name Myfembree for the first once-daily treatment for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women.
Why Choose Us as Your Relugolix API Manufacturer?
- Established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, Qingmu’s factory totally complies with environmental law in China, no risk to remove factory. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research. We are always ready to provide you with the best quality products.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) and also customer audits from Europe, USA and Japan, etc.
- Qingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.