Ruxolitinib Phosphate API
| CAS No. | 1092939-17-7 |
| Therapeutic Category | Oncology |
| Technology | Anti-Cancer/ Oncology |
| Dosage Form | Synthetic |
| Innovator Brand | Jakafi, Opzelura(USA) |
| Registration Status | China: I Others: DMF |
| Polymorph | TBD |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
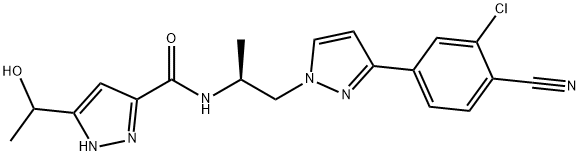
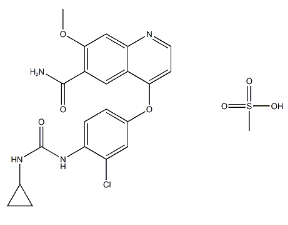
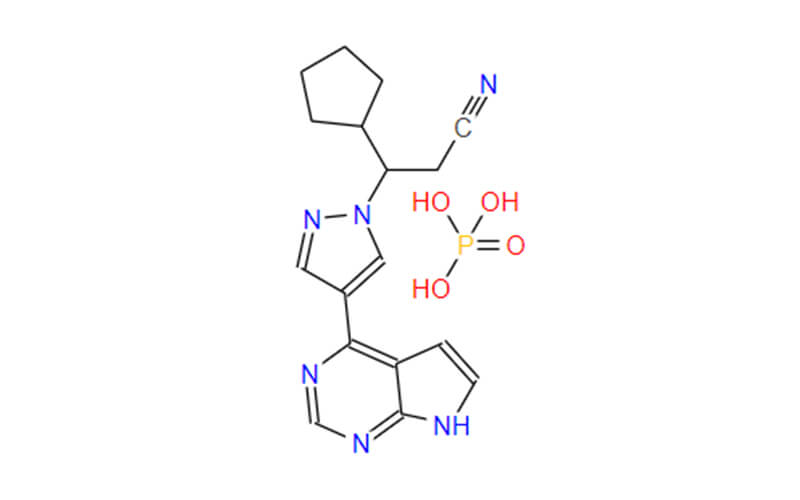
- Chemical Name: (3R)-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propionitrile phosphate
- Molecular Formula: C17H21N6O4P
- Molecular Weight:404.360
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Ruxolitinib API
- Ruxolitinib phosphate is a phosphate salt obtained by the reaction of ruxolitinib with one equivalent of phosphoric acid.
- Ruxolitinib API, formerly known as INCB018424 or INC424, is an anticancer drug and a Janus kinase (JAK) inhibitor. It is a potent and selective inhibitor of JAK1 and JAK2, which are tyrosine kinases involved in cytokine signaling and hematopoiesis. Myeloproliferative neoplasms, such as myelofibrosis and polycythemia vera, are often characterized by aberrant activation of the JAK-STAT pathway, leading to abnormal blood cell counts and thrombotic complications. By inhibiting JAK1 and JAK2, ruxolitinib works to block the dysregulated cell signaling pathways and prevents abnormal blood cell proliferation. Due to a large number of patients with myeloproliferative neoplasms who have JAK2 mutations, ruxolitinib was the first ATP-competitive inhibitor of JAK1 and JAK2 ever developed.
- Ruxolitinib was first approved for the treatment of adult patients with myelofibrosis by the FDA in 2011, followed by EMA‘s approval in 2012. In 2014, it was approved for the treatment of polycythemia vera in adults who have an inadequate response to or are intolerant of hydroxyurea, and in 2019, ruxolitinib was approved for use in steroid-refractory acute graft-versus-host disease in adults, and children. The topical formulation of ruxolitinib is used to treat atopic dermatitis and vitiligo. It is being investigated for other inflammatory skin conditions.
- Ruxolitinib has been investigated to treat patients with coronavirus disease 2019 (COVID-19) accompanied by severe systemic hyperinflammation. In phase II clinical trials, ruxolitinib improved chest computed tomography and improved recovery in patients with lymphopenia. However, phase III clinical trials later determined that ruxolitinib was inadequate in meeting its primary endpoint of reducing the number of hospitalized COVID-19 patients who experienced severe complications thus the drug was not approved as a treatment for COVID-19.
Why Choose Us as Your Ruxolitinib Manufacturer?
- Qingmu is a Ruxolitinib supplier, our Ruxolitinib API has been filed in China(DMF filed and listed on CDE’s website), and DMF in CTD format is available and can be supported for registration worldwide.
- Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, no risk to removing the factory. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- As a Ruxolitinib manufacturer, Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA), and MFDS(Korea) as well as customer audits from Europe, USA, Japan, etc.
- Qingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.