Understanding Lenvatinib’s Role in Cancer Treatment
Lenvatinib, also known by its brand name Lenvima, has emerged as a significant player in the fight against various cancers. This targeted therapy medication works by inhibiting specific molecules involved in tumor growth and spread. This article delves into the intricacies of lenvatinib, exploring its potential applications in cancer treatment, combination therapies, and side effect management.
Active Pharmaceutical Ingredient of lenvatinib
Lenvatinib API’s effectiveness stems from its targeted action on a broad spectrum of receptor tyrosine kinases (RTKs). These RTKs play a pivotal role in various cellular processes critical for tumor development and progression. Lenvatinib specifically inhibits the following key RTKs:
- Vascular endothelial growth factor receptors (VEGFRs) 1-3: These receptors are instrumental in angiogenesis, the process of new blood vessel formation. By inhibiting VEGFRs, lenvatinib restricts the blood supply to tumors, essentially starving them of oxygen and essential nutrients, thereby hindering their growth.
- Fibroblast growth factor receptors (FGFRs) 1-4: These receptors are involved in cell proliferation, survival, and migration. Inhibiting FGFRs disrupts these processes, hindering tumor growth and spread.
- Platelet-derived growth factor receptor alpha (PDGFRα): This receptor is involved in cell proliferation and migration, and its inhibition by lenvatinib contributes to the drug’s anti-tumor effects.
- Other targets: Lenvatinib’s spectrum of action extends beyond these core targets, also impacting additional signaling pathways involved in cancer progression, such as the RET and c-KIT proto-oncogenes.
Understanding Lenvatinib Mesylate
Lenvatinib mesylate is the salt form of the drug lenvatinib, a multi-kinase inhibitor used to treat various cancers. Converting the drug into a salt form offers several advantages:
- Enhanced stability: Salts can be more chemically stable than the parent drug, improving their shelf life and reducing the risk of degradation during storage and handling.
- Improved solubility: Salts are often more soluble in water than the parent drug, making them easier to formulate into liquid dosage forms like tablets or capsules for oral administration. This is crucial for lenvatinib, which has poor aqueous solubility in its pure form.
- Controlled release: Certain salt forms can be designed to release the drug slowly over time, potentially improving its efficacy and reducing the frequency of dosing.
In the case of lenvatinib mesylate, the mesylate salt specifically:
- Significantly increases the drug’s solubility in water, enabling its formulation into oral medications.
- Enhances the stability of lenvatinib, allowing for longer shelf life and better handling characteristics.
It’s important to note that the therapeutic effect of lenvatinib remains the same regardless of whether it’s administered in its pure form or as the mesylate salt. The salt form is simply a pharmaceutical strategy to improve the drug’s deliverability and handling properties.
Lenvatinib Mesylate as A Potential Treatment for Cancer
Clinical studies have demonstrated the efficacy of lenvatinib mesylate in prolonging progression-free survival and overall survival in patients with advanced or metastatic thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is typically used as a systemic therapy either alone or in combination with other anticancer agents, depending on the specific type and stage of cancer.
Lenvatinib’s targeted approach has garnered FDA approval for the treatment of several cancer types, including:
- Unresectable hepatocellular carcinoma (HCC): This is the most common type of primary liver cancer. Lenvatinib is used for the treatment of patients with unresectable HCC, meaning the tumor cannot be surgically removed.
- Radioactive iodine-refractory differentiated thyroid cancer (DTC): This type of thyroid cancer does not respond to treatment with radioactive iodine, a standard therapy for DTC. Lenvatinib is used in patients with locally recurrent or metastatic progressive DTC.
- Advanced renal cell carcinoma (RCC): This is a type of kidney cancer. Lenvatinib is used in combination with another medication called everolimus for the treatment of patients with advanced RCC.
Clinical trials are actively investigating the efficacy of lenvatinib in treating other types of cancer, including urothelial carcinoma, squamous cell carcinoma of the head and neck, and non-small cell lung cancer.
A Deeper Look into Lenvatinib for HCC Treatment
The approval of lenvatinib for HCC treatment was based on robust clinical trials demonstrating its efficacy. Compared to sorafenib, the previous standard treatment for advanced HCC, lenvatinib in combination with everolimus has shown:
- Improved Overall Survival (OS): Clinical trials evaluating lenvatinib in patients with advanced HCC have demonstrated improved overall survival compared to sorafenib, the previous standard of care. In these studies, patients receiving lenvatinib either as monotherapy or in combination with other agents experienced a statistically significant increase in overall survival. This means that patients lived longer when treated with lenvatinib compared to those treated with sorafenib.
- Extended Progression-Free Survival (PFS): Lenvatinib therapy has also been associated with extended progression-free survival compared to sorafenib. Progression-free survival refers to the length of time during and after treatment when a patient’s disease does not worsen. In clinical trials, patients treated with lenvatinib experienced a longer period of disease control compared to those treated with sorafenib. This suggests that lenvatinib may slow down the progression of HCC and provide patients with a longer period of stable disease.
- Superiority in Certain Subgroups: Subgroup analyses of clinical trial data have shown that lenvatinib may be particularly effective in certain patient populations. For example, lenvatinib has demonstrated efficacy in patients with advanced HCC who have previously received sorafenib treatment, as well as in those with Child-Pugh class A liver function. Additionally, lenvatinib has shown efficacy across various etiologies of HCC, including hepatitis B and hepatitis C virus infections.
Overall, the clinical evidence supporting the efficacy of lenvatinib in the treatment of HCC is compelling, with improvements in overall survival, progression-free survival, and tolerability compared to sorafenib. Lenvatinib represents an important addition to the treatment armamentarium for patients with advanced HCC and has the potential to improve outcomes and quality of life for individuals affected by this challenging disease.
Combination Therapies with Lenvatinib for Cancer Treatment
Lenvatinib not only as a single agent but also when combined with other therapeutic approaches. This synergistic strategy holds immense promise for enhancing treatment efficacy and overcoming resistance mechanisms.
Why Combination Therapies?
Combining lenvatinib with other drugs offers several advantages:
- Targeting multiple pathways: Cancer cells are complex and often employ various mechanisms to evade treatment. Combining lenvatinib with drugs targeting different pathways can create a more comprehensive attack, potentially leading to better tumor control.
- Overcoming resistance: Cancer cells can develop resistance to single-agent therapies. Combining drugs with different mechanisms of action can help prevent or delay the emergence of resistance, leading to more durable treatment responses.
- Improved efficacy: Combining lenvatinib with other drugs can potentially lead to a more potent anti-tumor effect compared to either therapy alone.

Current Landscape of Lenvatinib Combinations:
Lenvatinib is currently being investigated and used in combination with various drugs for different types of cancer, including:
- Hepatocellular carcinoma (HCC): Lenvatinib is approved in combination with everolimus for the treatment of unresectable HCC, demonstrating improved survival outcomes compared to single-agent therapy.
- Non-small cell lung cancer (NSCLC): The combination of lenvatinib with pembrolizumab, an immunotherapy drug, has shown promising results in advanced NSCLC, leading to its approval for this specific use.
- Endometrial cancer: Lenvatinib combined with pembrolizumab is being explored for the treatment of advanced endometrial cancer with encouraging early results.
- Other cancers: Ongoing clinical trials are evaluating the efficacy of lenvatinib combinations in various malignancies, including pancreatic cancer, colorectal cancer, and head and neck cancers.
Lenvatinib’s therapeutic potential is further amplified when combined with other cancer treatment modalities, such as chemotherapy or immunotherapy. This synergistic approach can potentially enhance the overall effectiveness of treatment by targeting different aspects of cancer growth and progression.
Management of Side Effects of Lenvatinib
While lenvatinib offers promising therapeutic benefits, its use may be accompanied by certain adverse effects that necessitate diligent management. Common side effects include hypertension, fatigue, diarrhea, decreased appetite, nausea, vomiting, proteinuria, and hand-foot syndrome. Close monitoring and proactive interventions are essential to mitigate these adverse events and ensure patient adherence to treatment.
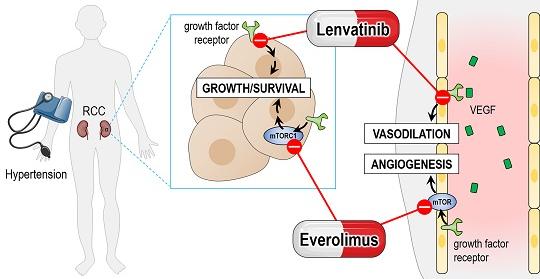
Medical interventions such as antihypertensive medications, antiemetics, antidiarrheals, and topical agents may be prescribed to alleviate specific side effects. Furthermore, dose adjustments or temporary interruptions may be warranted to manage toxicity while maintaining therapeutic efficacy.
Patient education and supportive care are integral components of managing side effects associated with lenvatinib therapy. Empowering patients with information about potential adverse effects, coping strategies, and available resources can enhance their ability to adhere to treatment and maintain their quality of life throughout the course of therapy.
Conclusion
Lenvatinib represents a significant advancement in the field of cancer therapeutics, offering a targeted approach to disrupt tumor growth and angiogenesis. Its versatility and potential for combination therapy make it a valuable asset in the armamentarium against various malignancies. As ongoing research continues to unravel the intricacies of lenvatinib’s mechanism of action and its optimal integration into treatment paradigms, it holds promise for improving patient outcomes and reshaping the landscape of cancer care. Effective management of side effects is paramount to optimizing treatment tolerability and ensuring the long-term success of lenvatinib-based therapies. Through collaborative efforts between clinicians, researchers, and patients, lenvatinib stands poised to make a meaningful impact in the fight against cancer.