Apalutamide: A Second-Generation NSAA for Non-Metastatic Castration-Resistant Prostate Cancer
Apalutamide, a second-generation nonsteroidal antiandrogen (NSAA), has emerged as a potent therapeutic agent for the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). Unlike its predecessors, apalutamide exhibits a unique binding affinity to the androgen receptor (AR), demonstrating efficacy in both AR-positive and AR-negative tumors. This article delves into the intricacies of apalutamide, exploring its active pharmaceutical ingredient (API), manufacturers, generic prospects, drug class comparison, side effects, and cost implications.
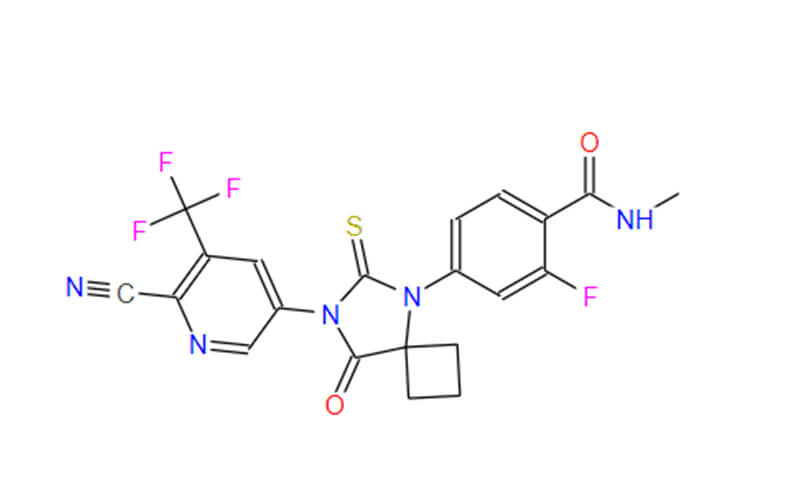
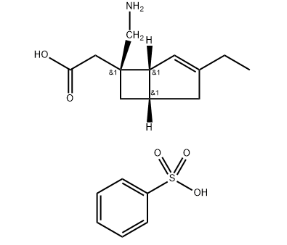
Apalutamide API: A Potent Steroid Receptor Antagonist
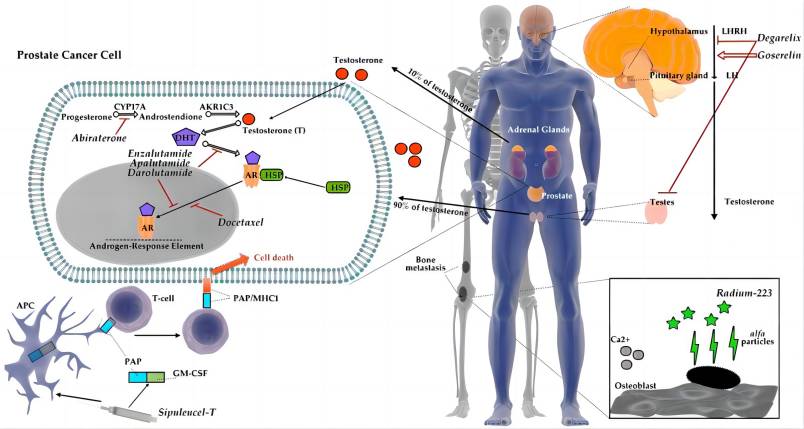
The therapeutic efficacy of apalutamide hinges upon its active pharmaceutical ingredient (API), (R)-N-(3-{[5-(difluoromethyl)-2-pyridinyl]oxy}-2-hydroxypropyl)-2,6-difluorobenzamide. This synthetic molecule, characterized by its intricate chemical structure, exhibits a high degree of binding affinity to the androgen receptor (AR). This targeted interaction disrupts the AR’s ability to bind and be activated by testosterone and dihydrotestosterone, the primary androgens fueling prostate cancer cell proliferation.
Several key structural features contribute to apalutamide’s potent antiandrogenic activity:
- Nonsteroidal structure: Unlike its steroidal predecessors, apalutamide lacks the characteristic four-ringed structure associated with conventional steroids. This structural divergence confers several advantages, including improved oral bioavailability and reduced risk of side effects associated with steroid-based therapies.
- Fluorine substitutions: The strategic positioning of fluorine atoms at the 2 and 6 positions of the benzamide ring enhances apalutamide’s binding affinity to the AR. These fluorine moieties increase the molecule’s lipophilicity, facilitating its penetration into the lipid-rich AR ligand-binding pocket, thereby competitively displacing testosterone and dihydrotestosterone.
- Hydroxypropyl and pyridinyl moieties: The hydroxypropyl and pyridinyl groups further contribute to apalutamide’s binding specificity by forming hydrogen bonds and stabilizing interactions with key amino acid residues within the AR ligand-binding pocket.
This precise binding interaction with the AR ultimately leads to a cascade of downstream effects, including:
- Inhibition of AR-mediated gene transcription: By preventing AR activation, apalutamide hinders the expression of genes crucial for prostate cancer cell growth and survival.
- Induction of apoptosis: The lack of AR-mediated signaling triggers programmed cell death in prostate cancer cells, leading to tumor shrinkage and disease control.
- Suppression of tumor angiogenesis: Apalutamide also exhibits anti-angiogenic properties, hindering the formation of new blood vessels necessary for tumor growth and metastasis.
Apalutamide Manufacturers: A Global Supply Chain Network
While Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, holds the commercialization rights for the finished drug product apalutamide, the intricate dance behind its production involves a broader network of players. At the heart of this ecosystem lies the active pharmaceutical ingredient (API), meticulously crafted by specialized organizations known as contract development and manufacturing organizations (CDMOs).
These CDMOs operate across diverse geographical regions, with a significant presence in China and India. This global distribution offers several advantages:
- Supply Chain Security: Diversifying manufacturing locations mitigates dependence on any single source, fostering a more resilient supply chain and ensuring consistent availability of the drug even in the face of unexpected disruptions.
- Cost Optimization: The geographical distribution of manufacturers introduces healthy competition, potentially driving down production costs and contributing to price variations based on regional regulations, labor costs, and raw material availability.
Among the prominent CDMOs contributing to apalutamide’s API production is Qingmu Pharmaceutical, a China-based company boasting extensive experience in developing and manufacturing complex APIs. Utilizing cutting-edge synthetic technologies and adhering to stringent quality control protocols, Qingmu plays a crucial role in bringing apalutamide to patients worldwide.
However, this intricate global network also presents challenges:
- Regulatory Compliance: Navigating the diverse regulatory landscapes of different countries can be complex for both CDMOs and pharmaceutical companies, potentially leading to delays and logistical hurdles.
- Quality Control Consistency: Ensuring consistent quality standards across a geographically dispersed network is paramount. Rigorous quality control measures and robust supply chain management practices are essential in maintaining the safety and efficacy of the final product.
Why the Apalutamide Price is So Expensive?
Apalutamide’s potent therapeutic action comes at a substantial cost. In the United States, the monthly price tag currently exceeds $7,000, placing a significant financial burden on patients and healthcare systems alike. This hefty price tag poses a major barrier to access, even for patients with insurance coverage, as co-pays and deductibles can further exacerbate financial hardship.
Several factors contribute to apalutamide’s high cost:
- High Research and Development (R&D) Costs: Developing a novel drug like apalutamide involves extensive research, clinical trials, and regulatory approvals, each incurring significant financial investments. These R&D costs are often factored into the initial pricing of the branded medication.
- Manufacturing Complexity: The intricate synthetic process and stringent quality control measures required for producing apalutamide’s API contribute to its production costs. Maintaining a globally dispersed manufacturing network can also introduce logistical complexity and additional overhead expenses.
- Market Exclusivity: As the sole commercial provider of apalutamide until the patent expiration, Janssen Pharmaceuticals possesses market exclusivity, allowing them to set the initial price based on their internal cost-recovery and profit objectives.
This high cost presents a major public health challenge, potentially limiting access to this potentially life-extending therapy for a significant portion of the patient population. The anticipated arrival of generic versions in 2027 offers a glimmer of hope for a more affordable treatment landscape.
The introduction of generics is expected to significantly reduce the cost of apalutamide through:
- Reduced R&D Costs: Generic manufacturers do not incur the initial R&D costs associated with developing the drug, allowing them to offer significantly lower prices compared to the branded product.
- Increased Competition: The presence of multiple generic manufacturers fosters healthy competition, further driving down prices and incentivizing cost-optimization strategies within the market.
- Negotiation Leverage: Increased patient and payer bargaining power with multiple generic options available can lead to more favorable pricing agreements and wider insurance coverage for the drug.
Apalutamide’s Generics Gambit: A Balancing Act between Affordability and Rigor
Looming on the horizon for apalutamide is the expiration of its patent in 2027, an event anticipated to trigger a seismic shift in the drug’s economic landscape. The influx of generic competitors is poised to disrupt the current market dominance of branded apalutamide, potentially resulting in:
- Substantial Price Reductions: The introduction of generics typically incurs significantly lower production costs due to streamlined processes and reduced research and development burdens. This cost advantage is expected to translate into substantial price reductions for patients, potentially making apalutamide treatment more accessible to a broader socioeconomic population.
- Increased Market Competition: The presence of multiple generic manufacturers fosters healthy competition, further driving down prices and incentivizing continual innovation and quality improvements within the market. This can benefit patients through enhanced access and potentially improved treatment options.
However, navigating the path to widespread availability of generic apalutamide is not without its challenges:
- Stringent Regulatory Hurdles: Generic drug approval processes are meticulously designed to ensure quality and efficacy equivalence to the original branded product. These rigorous testing and regulatory procedures, while crucial for patient safety, can introduce delays in the initial introduction of generics.
- Potential Quality Concerns: Maintaining consistent quality standards across various generic manufacturers within a diverse global market requires robust regulatory oversight and stringent quality control measures. Ensuring patient safety and drug efficacy becomes even more critical in a competitive environment with potentially lower production costs.

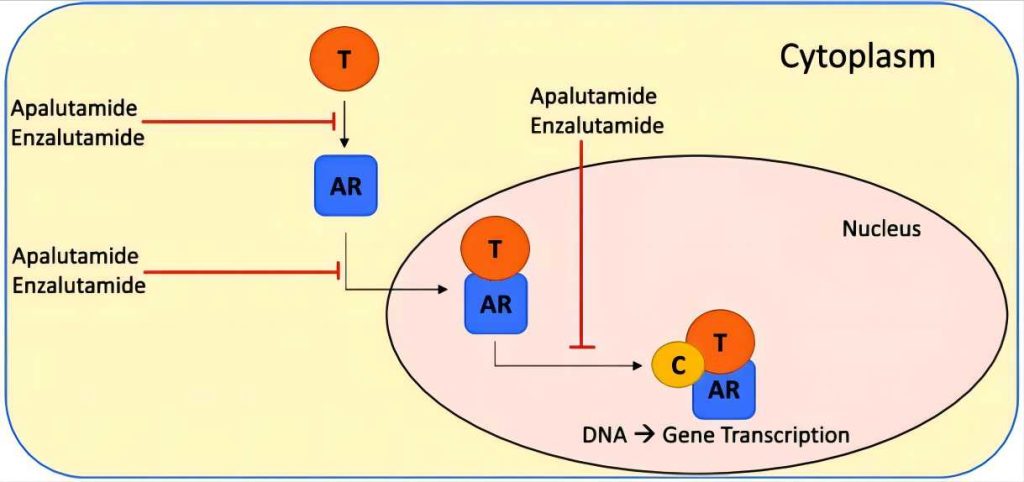
The Application of Apalutamide in the NSAA Arena
Apalutamide occupies a prominent position within the nonsteroidal antiandrogen (NSAA) drug class, sharing the landscape with fellow AR antagonists like enzalutamide and darolutamide. However, apalutamide distinguishes itself within this competitive arena through its unique pharmacological profile and clinical advantages:
- Superior Binding Affinity: Unlike its counterparts, apalutamide exhibits a distinct binding interaction with the AR, characterized by greater specificity and tighter binding at the ligand-binding pocket. This enhanced affinity translates to potent inhibition of AR signaling, potentially leading to improved clinical efficacy, particularly in tumors harboring AR mutations rendering them resistant to other NSAAs.
- Enhanced Clinical Spectrum: Studies suggest apalutamide’s efficacy extends beyond AR-wildtype tumors, demonstrating promising activity against AR-mutant tumors previously unresponsive to enzalutamide and darolutamide. This broader clinical spectrum positions apalutamide as a valuable therapeutic option for a wider range of patients with NM-CRPC.
- Favorable CNS Penetration: A significant concern with enzalutamide is its potential for central nervous system (CNS) penetration, leading to neurological side effects such as seizures. Apalutamide, on the other hand, exhibits minimal CNS penetration, significantly reducing the risk of these adverse events and improving patient tolerability.
- Favorable Safety Profile: While all NSAAs share a common side effect profile, apalutamide demonstrates a relatively lower incidence of certain adverse events compared to its counterparts. For instance, fatigue, a frequent complaint with enzalutamide, appears less prevalent with apalutamide.
- Emerging Synergistic Potential: Preclinical and early clinical data suggest promising synergies between apalutamide and other novel agents, including next-generation AR antagonists and immunotherapies. This opens up exciting possibilities for combinatorial treatment strategies to further improve outcomes for patients with NM-CRPC.
Side Effects of Apalutamide: A Risk-Benefit Analysis
While heralded for its efficacy in NM-CRPC, apalutamide, like any potent therapeutic agent, is not without its potential adverse effects. Understanding the side effect profile and implementing proactive risk management strategies are crucial for optimizing patient safety and treatment outcomes.
1. Common Adverse Events
- Fatigue: Fatigue tops the list of most frequent side effects, reported by approximately 30-40% of patients receiving apalutamide. This can manifest as a generalized lack of energy, decreased stamina, and difficulty performing daily activities.
- Hot flashes: Vasoactive effects of apalutamide can trigger hot flashes, characterized by sudden and intense sensations of heat, often accompanied by sweating and flushing. These occurrences can disrupt sleep and negatively impact quality of life.
- Hypertension: A rise in blood pressure is another common side effect, seen in approximately 10-20% of patients. Careful monitoring and potential adjustments in antihypertensive medications are necessary to manage this risk.
- Musculoskeletal pain: Myalgia and arthralgia, manifesting as muscle pain and joint discomfort, are reported by around 15-20% of patients. These symptoms can interfere with daily activities and may require symptomatic treatment.
2. Serious Adverse Events
- Seizures: While uncommon, apalutamide can elevate the risk of seizures, particularly in individuals with pre-existing seizure disorders or those taking concomitant medications known to lower the seizure threshold. Careful patient selection, pre-treatment screening for seizure risk factors, and close monitoring are essential for mitigating this potential risk.
- Falls and fractures: The combination of fatigue, dizziness, and musculoskeletal pain can increase the risk of falls and subsequent fractures in some patients. Implementing fall prevention strategies and bone health assessments can help minimize this risk.
- Hepatotoxicity: While rare, elevations in liver enzymes and potential liver injury have been reported with apalutamide use. Regular monitoring of liver function tests is crucial for early detection and management of any potential hepatotoxicity.
3. Risk Management Strategies
- Pre-treatment Assessment: Comprehensive patient history and medication review are crucial for identifying potential risk factors for adverse events, such as pre-existing conditions or concomitant medications that may potentiate side effects.
- Dose Monitoring and Adjustment: Careful dose titration and individualization based on patient tolerability and response are essential for minimizing adverse events while maintaining treatment efficacy.
- Proactive Symptom Management: Implementing supportive care measures such as fatigue management strategies or antihypertensive medications can mitigate the impact of common side effects and improve patient quality of life.
- Patient Education and Vigilance: Empowering patients with knowledge about potential side effects and encouraging prompt reporting of any concerning symptoms are crucial for early detection and intervention.
Conclusion
Apalutamide represents a significant advancement in the treatment of NM-CRPC, offering a potent and targeted approach with a distinct safety profile compared to earlier NSAAs. As the scientific community continues to delve deeper into its clinical potential and optimize its use in combination with other therapies, apalutamide is poised to play a pivotal role in improving the prognosis and quality of life for patients battling this challenging disease.