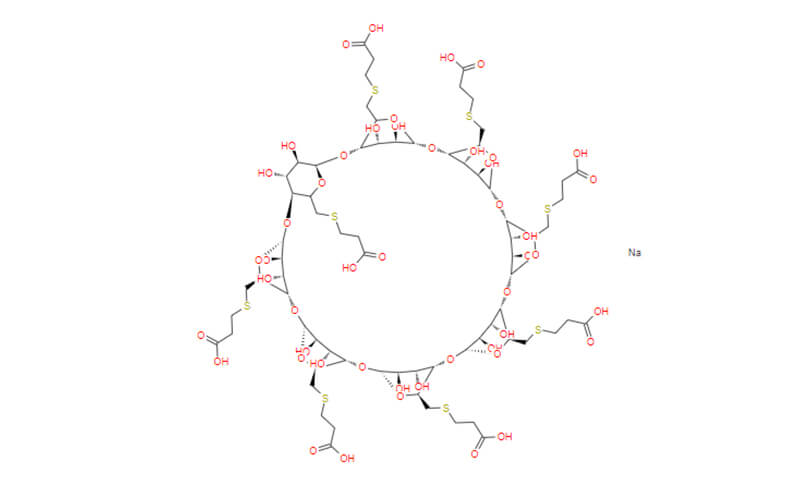
Sugammadex Sodium API
| CAS No. | 343306-79-6 |
| Therapeutic Category | Anesthetic & Analgesics |
| Technology | Synthetic |
| Dosage Form | Injection |
| Innovator Brand | Bridion(USA) |
| Registration Status | China: A Others: DMF |
| Polymorph | N/A |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
- Chemical Name: Sugammadex Sodium
- Molecular Formula: C72H104Na8O48S8
- Molecular Weight:2178.01000
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Sugammadex Sodium
- Sugammadex sodium is an organic sodium salt that is the octasodium salt of sugammadex. Used for reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. It has a role as a neuromuscular agent. It contains a sugammadex(8-).
- To facilitate tracheal intubation, mechanical ventilation, and surgical access, NMB agents are frequently used as non-anesthetic adjuncts in surgical procedures. Sugammadex is able to function as a pharmacologic sink of rocuronium and vecuronium, another non-depolarizing neuromuscular blocker, without the cardiovascular adverse effects experienced with reversal agents that directly interact with the cholinergic system. The γ-cyclodextrin has been designed to enhance the binding of the guest by incorporating acidic side chains to promote an electrostatic interaction with the positive nitrogen of the blocker. Starting with γ-cyclodextrin, these side chains are readily installed by first halogenating with iodine or bromine to provide a handle for nucleophilic displacement with either 3-mercaptopropionic acid in the presence of sodium hydride or with 3-mercaptopropionic acid methyl ester and cesium carbonate. The latter requires hydrolysis with sodium hydroxide to generate sugammadex sodium.
Why Choose Us as Your Sugammadex Manufacturer?
- Qingmu, as a Sugammadex supplier, our products have been approved in China(DMF filed and listed on CDE’s website), DMF in CTD format is available and can be supported for registration worldwide.
- Established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, Qingmu’s factory totally complies with environmental law in China, with no risk of removing the factory. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu is a sugammadex manufacturer that specializes in R&D and manufacturing of generic APIs and advanced intermediates. Our team has rich experience in patent challenges on crystalline form & synthesis processes, synthetic route development, scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA), and MFDS(Korea) as well as customer audits from Europe, USA, Japan, etc.
- We successfully exported our products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil. You can rest assured to choose us as your manufacturer.