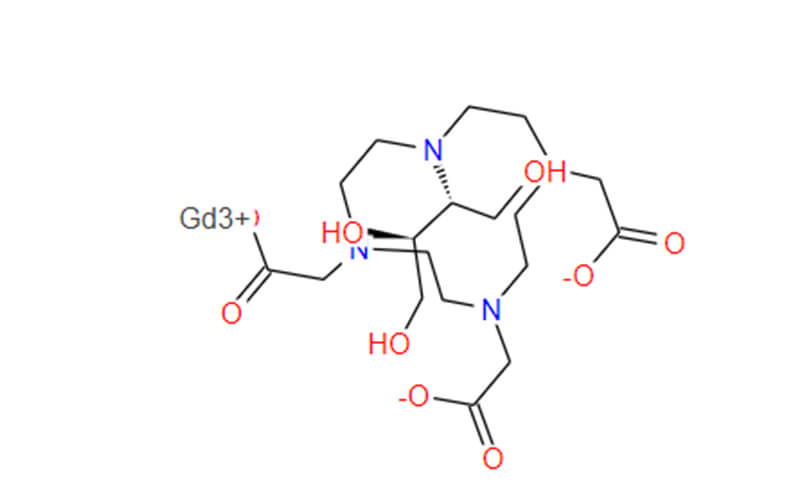
Gadobutrol API
| CAS No. | 138071-82-6 |
| Therapeutic Category | Contrast agent |
| Technology | Synthetic |
| Dosage Form | Injection |
| Innovator Brand | Gadovist(USA) |
| Registration Status | China: I Others: DMF |
| Polymorph | N/A |
| GMP | FDA(USA)/NMPA(China)/MFDS(Korea) approved |
| EHS | ISO 14001 & 45001 certified |
Product Description
- Grade: Active Pharmaceutical Ingredient(API)
- Chemical Name:1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid, 10-[(1R,2S)-2,3-dihydroxy-1-(hydroxymethyl)propyl]-, gadolinium salt (1:1)
- Molecular Formula: C18H31GdN4O9
- Molecular Weight: 604.710
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement
Applications of Gadobutrol API
- Intravenous gadobutrol is a second-generation extracellular non-ionic macrocyclic GBCA (gadolinium-based contrast agent) used in magnetic resonance imaging (MRI) in adults and children older than 2 years of age. It may help visualize and detect vascular abnormalities in the blood brain barrier (BBB) and central nervous system (CNS).
- In patients with impaired renal function, gadolinium-based contrast agents increase the risk of nephrogenic systemic fibrosis (NSF). A physician should be contacted if symptoms of NSF are encountered, such as dark or red patches on the skin; stiffness in joints; trouble moving, bending or straightening arms, hands, legs or feet; burning, itching, swelling, scaling, hardening and tightening of skin; pain in hip bones or ribs; or muscle weakness.
- Common adverse reactions that may be experienced include headache, nausea, feeling hot, abnormal taste, and warmth, burning or pain local to the injection site.
- General precautions should be taken in patients who are pregnant or breastfeeding, or who have a history of allergic reaction to contrast media, bronchial asthma or an allergic respiratory disorder.
Why Choose Us as Your Gadobutrol API Manufacturer?
- As a China Gadobutrol supplier, Qingmu’s products have been filed in China(DMF filed and listed on CDE’s website), DMF in CTD format is available and can be supported for registration in worldwide.
- Qingmu’s factory is established according to ICH/USFDA/EU/JAPAN/China regulations and current GMP, totally complies with environmental law in China, no risk to remove factory. A new API factory is under design and is predicted to be put into use in 2025.
- Qingmu’s team has rich experience in patent challenges on crystalline form & synthesis processes and also synthetic route development and scale-up & quality research.
- Qingmu’s lab is equipped with HPLC, GC, ICP-MS, GC-MS, CAD, microbalance, Malvern particle analyzer, etc. Qingmu passed the site inspection from NMPA(China), FDA(USA) and MFDS(Korea) and also customer audits from Europe, USA and Japan, etc. You can rest assured to choose us as your manufacturer.
- Qingmu successfully exported products to more than 40 countries including Japan, USA, Germany, Spain, the Netherlands, Russia, South Korea, and Brazil.